
SMOFKABIVEN LOW OSMO PERIPHERAL EMULSION FOR INFUSION

How to use SMOFKABIVEN LOW OSMO PERIPHERAL EMULSION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SmofKabiven Low Osmo Periférico emulsion for infusion
Read all of this leaflet carefully before you start using this medicine because it contains important information for you
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What SmofKabiven Low Osmo Periférico is and what it is used for
- What you need to know before you use SmofKabiven Low Osmo Periférico
- How to use SmofKabiven Low Osmo Periférico
- Possible side effects
- Storage of SmofKabiven Low Osmo Periférico
- Contents of the pack and other information
1. What SmofKabiven Low Osmo Periférico is and what it is used for
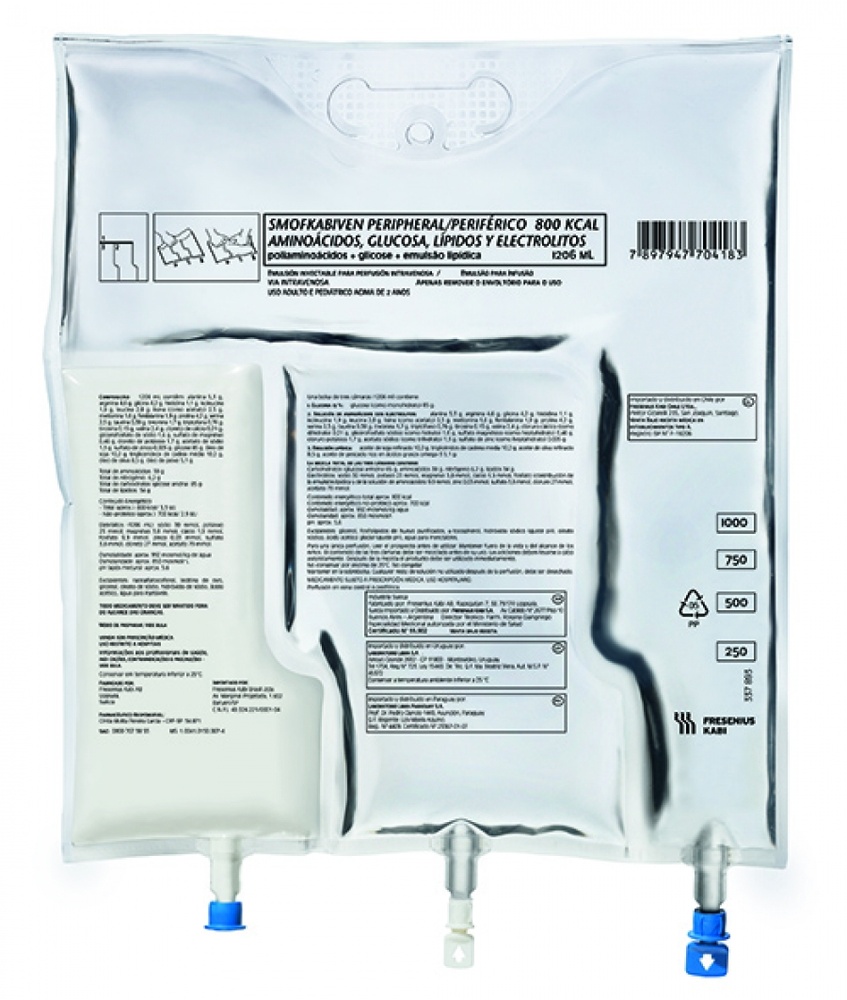
SmofKabiven Low Osmo Periférico is an emulsion for infusion that is administered into your blood through a drip (intravenous infusion). The product contains amino acids (components used in the formation of proteins), glucose (carbohydrates), lipids (fat), and salts (electrolytes), in a plastic bag and can be administered to adults and children from 2 years of age.
A healthcare professional will administer SmofKabiven Low Osmo Periférico when other forms of nutrition are not sufficient or not possible.
2. What you need to know before you use SmofKabiven Low Osmo Periférico
Do not use SmofKabiven Low Osmo Periférico:
- if you are allergic (hypersensitive) to the active substances or to any of the other components of this medicine (listed in section 6)
- if you are allergic to fish or egg
- if you are allergic to peanuts or soy, you should not use this product. SmofKabiven Low Osmo Periférico contains soybean oil
- if you have too much fat in your blood (hyperlipidemia)
- if you have severe liver impairment
- if you have blood coagulation problems (coagulation disorders)
- if your body has problems using amino acids
- if you have severe kidney disease without the possibility of dialysis
- if you are in acute shock
- if you have too much sugar in your blood (hyperglycemia) that is not controlled
- if you have high levels of salts (electrolytes) in your blood (serum) included in SmofKabiven Low Osmo Periférico
- if you have fluid in your lungs (acute pulmonary edema)
- if you have too much fluid in your body (overhydration)
- if you have untreated heart failure
- if you have a defect in your blood coagulation system (hemophagocytic syndrome)
- if you are in an unstable situation, such as after severe trauma, uncontrolled diabetes mellitus, acute heart attack, stroke, blood clot, metabolic acidosis (a condition that leads to too much acid in your blood), severe infection (sepsis), coma, and if you do not have enough fluid in your body (hypotonic dehydration)
- in newborn babies or children under 2 years of age
Warnings and precautions
Consult your doctor before starting to use SmofKabiven Low Osmo Periférico if you have:
- kidney problems
- diabetes mellitus
- pancreatitis (inflammation of the pancreas)
- liver problems
- hypothyroidism (thyroid problems)
- sepsis (severe infection)
If during infusion you experience fever, rash, swelling, difficulty breathing, chills, sweating, nausea, or vomiting, inform your healthcare professional immediately, as these symptoms could be caused by an allergic reaction or because you are receiving too much of the medicine.
Your doctor will need to regularly check your blood through liver function tests and other values.
Children and adolescents
SmofKabiven Low Osmo Periférico is not intended for newborn babies or children under 2 years of age. SmofKabiven Low Osmo Periférico can be administered to children from 2 to 18 years of age.
Using SmofKabiven Low Osmo Periférico with other medicines
Tell your doctor if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
There is no information on the use of SmofKabiven Low Osmo Periférico during pregnancy. SmofKabiven Low Osmo Periférico should only be administered to pregnant women if the doctor considers it absolutely necessary. The use of SmofKabiven Low Osmo Periférico may be considered during pregnancy if your doctor advises it.
No data are available on exposure in breastfeeding women.
The components and metabolites of parenteral nutrition such as SmofKabiven Low Osmo Periférico are excreted in human milk. Parenteral nutrition may be necessary during breastfeeding. SmofKabiven Low Osmo Periférico should only be administered to breastfeeding women when the doctor has weighed the potential risks and benefits.
Driving and using machines
This is not relevant, as this medicine is administered in the hospital.
3. How to use SmofKabiven Low Osmo Periférico
Follow exactly the administration instructions of this medicine given by your doctor. In case of doubt, consult your doctor again.
Your doctor will decide the dose for you individually depending on your body weight and situation. SmofKabiven Low Osmo Periférico will be administered by a healthcare professional.
If you use more SmofKabiven Low Osmo Periférico than you should
It is very unlikely that you will receive too much of the medicine, as SmofKabiven Low Osmo Periférico will be administered by a healthcare professional.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common(may affect up to 1 in 10 people): a slight increase in body temperature, inflammation in peripheral veins in connection with the injection site.
Uncommon(may affect up to 1 in 100 people): high levels of liver components in the blood (plasma), loss of appetite, nausea, vomiting, chills, dizziness, and headache.
Rare(may affect up to 1 in 1000 people): low or high blood pressure, difficulty breathing, rapid heart rate (tachycardia). Hypersensitivity reactions (which can cause symptoms such as swelling, fever, drop in blood pressure, skin rash, hives, redness, headache). Sensations of cold and heat. Paleness. Blue discoloration of the lips and skin (due to lack of oxygen in the blood). Pain in the neck, back, bones, chest, and lumbar region.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SmofKabiven Low Osmo Periférico
Keep this medicine out of the sight and reach of children.
Store in the outer bag. Do not store above 25°C. Do not freeze.
Do not use this medicine after the expiry date which is stated on the label of the bag and the carton. The expiry date is the last day of the month stated.
6. Container Contents and Additional Information
SmofKabiven Low Osmo Peripherally contains
The active substances are | g per 1,000 ml |
Glucose (as monohydrate) | 68 |
Alanine | 3.5 |
Arginine | 3.0 |
Glycine | 2.8 |
Histidine | 0.75 |
Isoleucine | 1.3 |
Leucine | 1.9 |
Lysine (as acetate) | 1.7 |
Methionine | 1.1 |
Phenylalanine | 1.3 |
Proline | 2.8 |
Serine | 1.6 |
Taurine | 0.25 |
Threonine | 1.1 |
Tryptophan | 0.50 |
Tyrosine | 0.10 |
Valine | 1.6 |
Calcium chloride (as dihydrate) | 0.14 |
Sodium glycerophosphate (as hydrate) | 1.0 |
Magnesium sulfate (as heptahydrate) | 0.30 |
Potassium chloride | 1.1 |
Sodium acetate (as trihydrate) | 0.85 |
Zinc sulfate (as heptahydrate) | 0.0032 |
Refined soybean oil | 11 |
Medium-chain triglycerides | 11 |
Refined olive oil | 8.8 |
Fish oil rich in omega-3 fatty acids | 5.3 |
The other components are: glycerol, purified egg phospholipids, all-rac-α-tocopherol, sodium hydroxide (pH adjustment), sodium oleate, glacial acetic acid (pH adjustment), and water for injectable preparations.
Appearance of the Product and Container Contents
The glucose and amino acid solutions are transparent, colorless, or slightly yellow and free of particles. The lipid emulsion is white and homogeneous.
Container Sizes:
1 x 850 ml, 5 x 850 ml
1 x 1,400 ml, 4 x 1,400 ml
1 x 1,950 ml, 4 x 1,950 ml
1 x 2,500 ml, 3 x 2,500 ml
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Fresenius Kabi España S.A.U.
C/ Marina 16-18.
08005 Barcelona (Spain)
Manufacturer
Fresenius Kabi AB, SE-751 74 Uppsala, Sweden
This Medicinal Product is Authorized in the Member States of the European Economic Area under the Following Names:
Member State Name | Medicinal Product Name |
Austria | SmofKabiven Low Osmo peripher Emulsion zur Infusion |
Belgium | SmofKabiven Low Osmo Perifeer Smofkabiven Low Osmo Périphérique SmofKabiven Low Osmo Peripher |
Bulgaria | ??????????? ??? ???? ????????? ?????????? ??????? |
Croatia | SmofKabiven Low Osmo Peripheral |
Cyprus | SmofKabiven Low Osmo Peripheral |
Czech Rep. | SmofKabiven Low Osmo Peripheral |
Denmark | SmofKabiven Low Osmo Peripheral |
Estonia | SmofKabiven Low Osmo Peripheral |
Finland | SmofKabiven Low Osmo Peripheral |
Germany | SmofKabiven Low Osmo peripher Emulsion zur Infusion |
Greece | SmofKabiven Low Osmo Peripheral |
Hungary | SmofKabiven Low Osmo Peripheral |
Iceland | SmofKabiven Low Osmo Peripheral |
Ireland | SmofKabiven Low Osmo Peripheral |
Latvia | SmofKabiven Low Osmo Peripheral |
Lithuania | SmofKabiven Low Osmo Peripheral |
Luxembourg | SmofKabiven Low Osmo peripher Emulsion zur Infusion |
Netherlands | SmofKabiven Low Osmo Perifeer |
Norway | SmofKabiven Low Osmo Peripheral |
Poland | SmofKabiven Low Osmo Peripheral |
Portugal | SmofKabiven Low Osmo Peripheral |
Romania | SmofKabiven Low Osmo Peripheral emulsie perfuzabila |
Slovakia | SmofKabiven Low Osmo Peripheral |
Slovenia | SmofKabiven Peripheral Low Osmo emulzija za infundiranje |
Spain | SmofKabiven Low Osmo Periférico. |
Sweden | SmofKabiven Low Osmo Peripheral |
United Kingdom | SmofKabiven Low Osmo Peripheral |
Date of the Last Revision of this Leaflet: July 2023
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Special Warnings and Precautions for Use
To avoid the risks associated with too rapid infusion rates, it is recommended to use continuous and well-controlled infusion, if possible using an infusion pump.
Since the use of a peripheral vein is associated with a high risk of infection, strict aseptic precautions should be taken to avoid any contamination during catheter insertion and handling.
Serum glucose, electrolytes, and osmolarity, as well as fluid balance, acid-base balance, and liver enzyme levels, should be monitored.
Upon any sign or symptom of anaphylactic reaction (such as fever, shivering, skin rash, or dyspnea), the infusion should be immediately discontinued.
SmofKabiven Low Osmo Peripherally should not be administered simultaneously with blood in the same infusion equipment due to the risk of pseudoagglutination.
Thrombophlebitis may occur if peripheral veins are used for infusion. The catheter insertion site should be checked daily for local signs of thrombophlebitis.
Method of Administration
Intravenous route, infusion in a peripheral or central vein.
To provide complete parenteral nutrition, oligoelements, vitamins, and possibly electrolytes (taking into account the electrolytes already present in SmofKabiven Low Osmo Peripherally) should be added to SmofKabiven Low Osmo Peripherally, according to the patient's needs.
Posology
Adults
Dosage:
The dose range of 20 ml - 40 ml of SmofKabiven Low Osmo Peripherally /kg body weight/day corresponds to 0.08-0.16 g nitrogen/kg body weight/day (0.5-1.0 g amino acids/kg body weight/day) and 14-29 kcal/kg body weight/day of total energy (12-25 kcal/kg body weight/day of non-protein energy).
Infusion Rate
The maximum infusion rate for glucose is 0.25 g/kg body weight/h, for amino acids 0.1 g/kg body weight/h, and for lipids 0.15 g/kg body weight/h.
The infusion rate should not exceed 3.7 ml/kg body weight/hour (corresponding to 0.25 g glucose, 0.09 g amino acids, and 0.13 g lipids/kg body weight/h). The recommended infusion period is 12-24 hours.
Maximum Daily Dose
The maximum daily dose varies with the patient's clinical situation and may even change from day to day. The recommended maximum daily dose is 40 ml/kg body weight/day.
Pediatric Population
Children (2-11 years)
Dosage:
The dose of up to 40 ml/kg body weight/day should be adjusted regularly according to the pediatric patient's requirements, which vary more than in adult patients.
Infusion Rate:
The maximum recommended infusion rate is 4.0 ml/kg body weight/h (corresponding to 0.10 g amino acids /kg/h, 0.27 g glucose/kg/h, and 0.14 g lipids/kg/h). At the maximum recommended infusion rate, infusion periods greater than 10 hours should not be used, except in exceptional cases and under close monitoring.
The recommended infusion period is 12-24 hours.
Maximum Daily Dose:
The maximum daily dose varies with the patient's clinical condition and may even change from day to day. The maximum daily dose is 40 ml/kg body weight/day.
Adolescents (12-18 years)
SmofKabiven Low Osmo Peripherally can be used in adolescents in the same way as in adults.
Disposal Precautions
Do not use if the container is damaged.
Use only if the amino acid and glucose solutions are transparent and colorless or slightly yellow, and if the lipid emulsion is white and homogeneous. The contents of the three separate chambers should be mixed before use and before making any additions through the additive port. After opening the peel-type seals, the bag should be inverted several times to ensure a homogeneous mixture, which does not show evidence of phase separation.
For single use. Any remaining mixture should be discarded after infusion.
Compatibility
Compatibility data are available for the products Dipeptiven, Supliven, Vitalipid Adults, Soluvit (lyophilized), and Glycophos in defined quantities and generic sodium or potassium solutions in defined concentrations. When adding sodium, potassium, or phosphate, the amounts already present in the bag should be taken into account according to the patient's clinical needs. The generated data support additions to the activated bag according to the following table:
Volume | |
SmofKabiven Low Osmo Peripherally | 850 ml, 1,400 ml, 1,950 ml, and 2,500 ml |
Additive | |
Dipeptiven | 0 - 300 ml |
Supliven | 0 - 10 ml |
Soluvit (lyophilized) | 0 - 1 vial |
Vitalipid Adults | 0 - 10 ml |
Electrolyte Interval* | |
Sodium | ≤ 150 mmol/l |
Potassium | ≤ 150 mmol/l |
Phosphate (Glycophos) | ≤ 15 mmol/l |
- Including the amounts present in the bag
Note: This table indicates compatibility. It is not a dosage guideline.
Additions should be made aseptically.
Validity Period after Mixing
The physical and chemical stability of the mixed three-chamber bag has been demonstrated for 36 hours at 25°C. From a microbiological point of view, the product should be used immediately. If not used immediately, the storage time before use and storage conditions are the responsibility of the user and should not normally exceed 24 hours at 2-8°C.
Validity Period after Mixing with Additives
From a microbiological point of view, the product should be used immediately after making additions. If not used immediately, the storage time before use and storage conditions are the responsibility of the user. The storage time should not normally exceed 24 hours at 2-8°C.
Instructions for the Use of SmofKabiven Low Osmo Peripherally
The Bag
850 ml, 1,400 ml, 1,950 ml, 2,500 ml
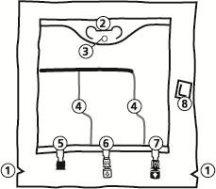
- Notches on the overbag
- Handle
- Hole for hanging the bag
- Breakable seals
- Blind port (used only during manufacturing)
- Additive port
- Infusion port
- Oxygen absorber
- Opening the Overbag
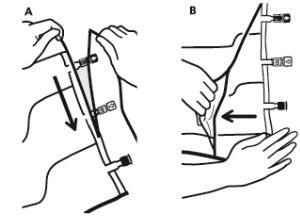
- To remove the overbag, hold it in a horizontal position and tear along the notch towards the ports along the top edge (A)
- Then, simply tear along the container; separate the overbag and discard it along with the oxygen absorber (B).
- Mixing
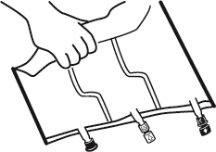
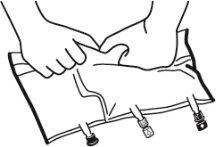
- Place the bag on a flat surface.
- Roll the bag from the hanger part towards the port part, first with the right hand and then applying constant pressure with the left hand until the vertical seals have opened. The vertical peel seals open due to the liquid pressure. The peel seals can also be opened before removing the overbag.
Note:The liquids mix easily even if the horizontal seal remains closed.
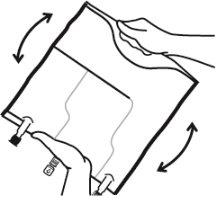
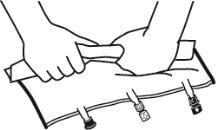
- Mix the contents of the three chambers by inverting the bag three times until the components are completely mixed.
- Final Preparation:
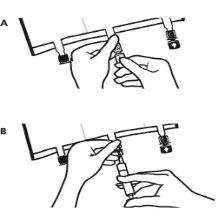
- Place the bag back on a flat surface. Just before injecting additives, break the white additive port by the arrow mark (A).
Note:The additive port membrane is sterile.
- Hold the base of the additive port. Insert the needle, inject the additives (of known compatibility) through the center of the injection point (B).
- Mix completely between each addition, inverting the bag three times. Use syringes with 18-23 gauge needles and a maximum length of 40 mm.
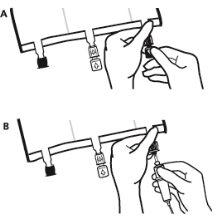
- Just before inserting the infusion set, break the blue infusion port by the arrow mark (A).
Note:The infusion port membrane is sterile.
- Use non-vented infusion equipment or close the air inlet of the vented equipment.
- Hold the base of the infusion port.
- Insert the spike through the infusion port. The spike should be fully inserted to ensure its retention.
Note:The inside of the infusion port is sterile.
- Hanging the Bag
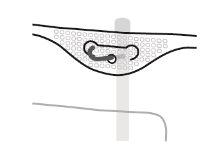
- Hang the bag by the ring under the hanger
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SMOFKABIVEN LOW OSMO PERIPHERAL EMULSION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for SMOFKABIVEN LOW OSMO PERIPHERAL EMULSION FOR INFUSION
Discuss questions about SMOFKABIVEN LOW OSMO PERIPHERAL EMULSION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















