SKYRIZI 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use SKYRIZI 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Skyrizi 75 mg Solution for Injection in Pre-filled Syringe
risankizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Skyrizi and what is it used for
- What you need to know before you use Skyrizi
- How to use Skyrizi
- Possible side effects
- Storage of Skyrizi
- Contents of the pack and other information
- Instructions for use
1. What is Skyrizi and what is it used for
Skyrizi contains the active substance risankizumab.
Skyrizi is used to treat the following inflammatory diseases:
- Plaque psoriasis
- Psoriatic arthritis
How Skyrizi works
This medicine works by blocking a protein in the body called “IL-23” that causes inflammation.
Plaque psoriasis
Skyrizi is used to treat moderate to severe plaque psoriasis in adults. Skyrizi reduces inflammation and can help reduce symptoms of plaque psoriasis such as itching, scratching, pain, redness, and flaking.
Psoriatic arthritis
Skyrizi is used to treat psoriatic arthritis in adults. Psoriatic arthritis is a disease that causes inflammation of the joints and psoriasis. If you have active psoriatic arthritis, you may first be given other medicines. If these medicines do not work well enough, you will be given Skyrizi alone or in combination with other medicines to treat your psoriatic arthritis.
Skyrizi reduces inflammation and can help reduce pain, stiffness, and swelling in your joints and around them, pain and stiffness in your spine, psoriatic skin rash, and nail damage due to psoriasis, as well as slow down damage to the bone and cartilage of your joints. These effects can make it easier for you to perform daily activities, reduce fatigue, and improve your quality of life.
2. What you need to know before you use Skyrizi
Do not use Skyrizi
- if you are allergic to risankizumab or any of the other ingredients of this medicine (listed in section 6).
- if you have an infection that your doctor thinks is important, for example, active tuberculosis.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before and during treatment with Skyrizi:
- if you have a current infection or if you have an infection that keeps coming back.
- if you have tuberculosis (TB).
- if you have recently received or are scheduled to receive a vaccine. Certain vaccines should not be given during treatment with Skyrizi.
It is important to keep a copy of the batch number of Skyrizi.
Each time you receive a new pack of Skyrizi, write down the date and batch number (which appears on the pack after “Batch”) and keep this information in a safe place.
Allergic reactions
Talk to your doctor or seek medical attention immediately if you notice any signs of an allergic reaction while receiving Skyrizi, such as:
- difficulty breathing or swallowing
- swelling of the face, lips, tongue, or throat
- intense itching of the skin, with a red rash or hives
Children and adolescents
Skyrizi is not recommended for children and adolescents under 18 years of age, as it has not been studied in this age group.
Other medicines and Skyrizi
Tell your doctor, pharmacist, or nurse:
- if you are using, have recently used, or might use any other medicines.
- if you have been vaccinated recently or are scheduled to be vaccinated. Certain vaccines should not be given during treatment with Skyrizi.
If in doubt, talk to your doctor, pharmacist, or nurse before using Skyrizi and during treatment.
Pregnancy, contraception, and breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. You must do this because it is not known how this medicine will affect your baby.
If you are a woman who can become pregnant, you must use contraception while being treated with this medicine and for at least 21 weeks after your last dose of Skyrizi.
If you are breastfeeding or plan to breastfeed, consult your doctor before using this medicine.
Driving and using machines
Skyrizi is unlikely to affect your ability to drive and use machines.
Skyrizi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe, which is essentially “sodium-free”.
3. How to use Skyrizi
Follow exactly the administration instructions for this medicine given by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
This medicine is given as 2 injections under the skin (called “subcutaneous injections”).
How much Skyrizi to use
The dose is 150 mg given as two 75 mg injections.
How much? | When? | |
1st dose | 150 mg (twoinjections of 75 mg) | When your doctor tells you |
2nd dose | 150 mg (twoinjections of 75 mg) | 4 weeks after the 1st dose |
Following doses | 150 mg (twoinjections of 75 mg) | Every 12 weeks from the 2nd dose |
You and your doctor, pharmacist, or nurse will decide if you can inject this medicine yourself. Do not inject this medicine yourself unless your doctor, pharmacist, or nurse has taught you how to do it. It is also possible that a caregiver who has learned how to do it can give you the injections.
Read section 7 “Instructions for use” at the end of this leaflet before giving yourself the Skyrizi injection.
If you use more Skyrizi than you should
If you have used more Skyrizi than you should or have given yourself a dose earlier than prescribed, talk to your doctor.
If you forget to use Skyrizi
If you forget to give yourself a dose of Skyrizi, inject a dose as soon as you remember. If in doubt, talk to your doctor.
If you stop using Skyrizi
Do not stop using Skyrizi without talking to your doctor first. If you stop treatment, your symptoms may come back.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Talk to your doctor or seek medical attention immediately if you have any symptoms of a serious infection, such as:
- fever, flu-like symptoms, night sweats
- feeling tired or having trouble breathing, persistent cough
- heat, redness, and pain in the skin or a painful skin rash with blisters
Your doctor will decide if you can continue using Skyrizi.
Other side effects
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects.
Very common:may affect more than 1 in 10 people
- upper respiratory tract infections with symptoms such as sore throat and nasal congestion
Common:may affect up to 1 in 10 people
- feeling tired
- fungal skin infection
- reactions at the injection site (such as redness or pain)
- itching
- headache
- rash
- eczema
Uncommon:may affect up to 1 in 100 people
- small red bumps on the skin
- hives (urticaria)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Skyrizi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the syringe and on the outer carton after EXP.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the pre-filled syringes in the original packaging to protect them from light.
Do not use this medicine if the liquid is cloudy or contains large flakes or particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Skyrizi Composition
- The active ingredient is risankizumab. Each prefilled syringe contains 75 mg of risankizumab in 0.83 ml of solution.
- The other components are disodium succinate hexahydrate, succinic acid, sorbitol, polysorbate 20, and water for injectable preparations.
Product Appearance and Container Contents
Skyrizi is a clear, colorless to slightly yellowish liquid contained in a prefilled syringe with a needle guard. The liquid may contain tiny, transparent or white particles.
Each container contains 2 prefilled syringes and 2 alcohol-impregnated wipes.
Marketing Authorization Holder
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Ludwigshafen
Germany
Manufacturer
AbbVie S.r.l.
04011 Campoverde di Aprilia
(Latina)
Italy
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien AbbVie SA Tel: +32 10 477811 | Lithuania AbbVie UAB Tel: +370 5 205 3023 |
| Luxembourg/Luxemburg AbbVie SA Belgique/Belgien Tel: +32 10 477811 |
Czech Republic AbbVie s.r.o. Tel: +420 233 098 111 | Hungary AbbVie Kft. Tel: +36 1 455 8600 |
Denmark AbbVie A/S Tlf: +45 72 30-20-28 | Malta V.J.Salomone Pharma Limited Tel: +356 22983201 |
Germany AbbVie Deutschland GmbH & Co. KG Tel: 00800 222843 33 (toll-free) Tel: +49 (0) 611 / 1720-0 | Netherlands AbbVie B.V. Tel: +31 (0)88 322 2843 |
Estonia AbbVie OÜ Tel: +372 623 1011 | Norway AbbVie AS Tlf: +47 67 81 80 00 |
Greece AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Τηλ: +30 214 4165 555 | Austria AbbVie GmbH Tel: +43 1 20589-0 |
Spain AbbVie Spain, S.L.U. Tel: +34 91 384 09 10 | Poland AbbVie Sp. z o.o. Tel: +48 22 372 78 00 |
France AbbVie Tél: +33 (0) 1 45 60 13 00 | Portugal AbbVie, Lda. Tel: +351 (0)21 1908400 |
Croatia AbbVie d.o.o. Tel: +385 (0)1 5625 501 | Romania AbbVie S.R.L. Tel: +40 21 529 30 35 |
Ireland AbbVie Limited Tel: +353 (0)1 4287900 | Slovenia AbbVie Biofarmacevtska družba d.o.o. Tel: +386 (1)32 08 060 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia AbbVie s.r.o. Tel: +421 2 5050 0777 |
Italy AbbVie S.r.l. Tel: +39 06 928921 | Finland AbbVie Oy Puh/Tel: +358 (0)10 2411 200 |
Cyprus Lifepharma (Z.A.M.) Ltd Τηλ: +357 22 34 74 40 | Sweden AbbVie AB Tel: +46 (0)8 684 44 600 |
Latvia AbbVie SIA Tel: +371 67605000 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu/, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
Detailed and updated information about this product is available below or on the outer packaging by scanning the QR code using a smartphone. The same information is also available on the following website: www.skyrizi.eu
QR Code to be included
To request a copy of this leaflet in
- Instructions for Use
Read the entire section 7 before using Skyrizi
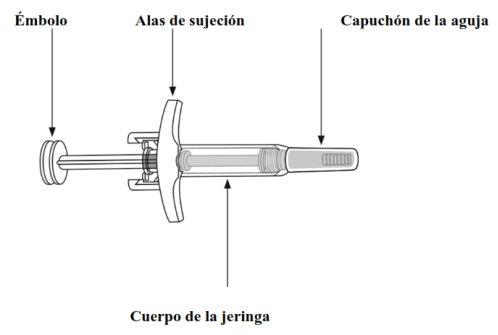
Important Information You Should Know Before Injecting Skyrizi
- You must have received training on how to inject Skyrizi before administering an injection. If you need help, consult your doctor, pharmacist, or nurse.
- Mark the dates on a calendar to know when it's time to inject Skyrizi.
- Keep Skyrizi in its original packaging to protect the medication from light until the time of use.
- Do notinject the medication if the liquid is cloudy or contains flakes or large particles. The liquid should be clear to slightly yellowish and may contain tiny, transparent or white particles.
- Do notshake the syringe.
- Wait to remove the needle cap until the moment before injection.
Return this medication to the pharmacy
- after the expiration date (EXP) indicated
- if the liquid has been frozen at any time (even if it has been thawed)
- if the syringe has been dropped or damaged
- if the paper cover of the tray is not present or is broken.
For a more comfortable injection: Remove the box from the refrigerator and let it sit at room temperature, away from direct sunlight, for 15 to 30 minutesbefore injection.
- Skyrizi should not be heated in any other way (e.g., in a microwave or in hot water).
- Keep the syringes in the box until the time of injection.
Follow these steps each time you use Skyrizi
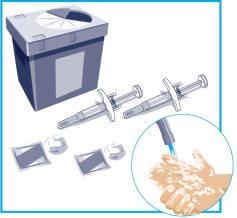
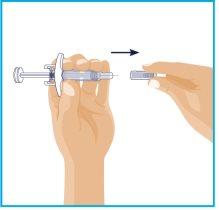
STEP1
| On a smooth and clean surface, place the following:
Wash and dry your hands. Start with a syringe for the first injection. To administer the full dose, it is necessary to give 2consecutive injections. |
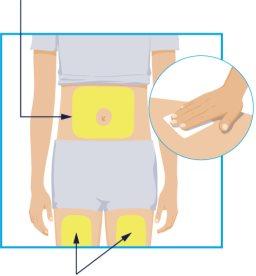
STEP2 Injection Sites
Injection Sites | Choose one of these 3 areas for injection:
When using the second syringe, you must inject at least 3 cm away from the site of the first injection. Do notinject in the same place. Before each injection, clean the injection site with an alcohol-impregnated wipe using circular motions.
|
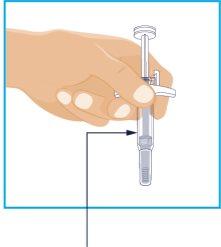
STEP3
Inspect the Liquid | Hold the syringe with the needle covered by the cap facing down as shown. Check the liquid in the syringe.
|
STEP4
| Remove the needle cap:
|
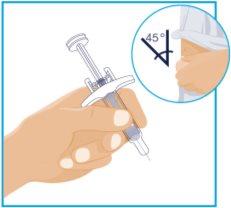
STEP5
| Hold the syringe body with one hand between the thumb and index finger, as if holding a pencil. Gently pinch the cleaned skin area with the other hand and hold it firmly. Insert the needle into the skin at an approximate angle of 45 degrees, with a short and quick motion. Keep the syringe fixed at the same angle. |
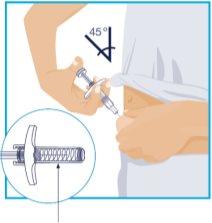
STEP6
Needle Protector | Slowly push the plunger until it reaches the end, until all the liquid has been injected. Slowly remove the needle from the skin, keeping the syringe at the same angle. Slowly release the thumb from the plunger. The needle protector will then cover the needle.
Press a cotton ball or gauze over the injection site and hold for 10 seconds. Do notrub the skin at the injection site. There may be slight bleeding at the injection site. This is normal. |
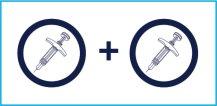
STEP7
2 Injections are Needed | To administer the full dose, it is necessary to give two consecutive injections.
|
STEP8
| Discard the used syringes in a special waste container immediately after use.
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SKYRIZI 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 150 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: INJECTABLE, 150 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: INJECTABLE, 180 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription required
Online doctors for SKYRIZI 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about SKYRIZI 75 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions