SKYRIZI 150 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use SKYRIZI 150 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Skyrizi 150mg solution for injection in pre-filled syringe
risankizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Skyrizi and what is it used for
- What you need to know before you use Skyrizi
- How to use Skyrizi
- Possible side effects
- Storage of Skyrizi
- Contents of the pack and further information
- Instructions for use
1. What is Skyrizi and what is it used for
Skyrizi contains the active substance risankizumab.
Skyrizi is used to treat the following inflammatory diseases:
- Plaque psoriasis
- Psoriatic arthritis
How Skyrizi works
This medicine works by blocking a protein in the body called “IL-23” that causes inflammation.
Plaque psoriasis
Skyrizi is used to treat moderate to severe plaque psoriasis in adults. Skyrizi reduces inflammation and can help reduce symptoms of plaque psoriasis such as itching, pain, redness, and flaking.
Psoriatic arthritis
Skyrizi is used to treat psoriatic arthritis in adults. Psoriatic arthritis is a disease that causes inflammation of the joints and psoriasis. If you have active psoriatic arthritis, you may first be given other medicines. If these medicines do not work well enough, you will be given Skyrizi alone or in combination with other medicines to treat your psoriatic arthritis.
Skyrizi reduces inflammation and can help reduce pain, stiffness, and swelling in your joints and around them, pain and stiffness in your spine, psoriatic skin rash, and nail damage due to psoriasis, as well as slow down damage to the bones and cartilage of your joints. These effects can make it easier for you to perform daily activities, reduce fatigue, and improve your quality of life.
2. What you need to know before you use Skyrizi
Do not use Skyrizi
- if you are allergic to risankizumab or any of the other ingredients of this medicine (listed in section 6).
- if you have an infection that your doctor thinks is important, for example, active tuberculosis.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Skyrizi and during treatment:
- if you have a current infection or if you have an infection that keeps coming back.
- if you have tuberculosis (TB).
- if you have recently received or are scheduled to receive a vaccine. Certain vaccines should not be given during treatment with Skyrizi.
It is important to keep a copy of the batch number of Skyrizi.
Each time you receive a new pack of Skyrizi, write down the date and batch number (which appears on the pack after “Batch”) and keep this information in a safe place.
Allergic reactions
Talk to your doctor or seek medical attention right away if you notice any signs of an allergic reaction while receiving Skyrizi, such as:
- difficulty breathing or swallowing
- swelling of the face, lips, tongue, or throat
- severe itching of the skin, with a red rash or hives
Children and adolescents
Skyrizi is not recommended for children and adolescents under 18 years of age, as it has not been studied in this age group.
Other medicines and Skyrizi
Tell your doctor, pharmacist, or nurse:
- if you are using, have recently used, or might use any other medicines.
- if you have recently been vaccinated or are scheduled to be vaccinated. Certain vaccines should not be given during treatment with Skyrizi.
If in doubt, talk to your doctor, pharmacist, or nurse before using Skyrizi and during treatment.
Pregnancy, contraception, and breastfeeding
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. You must do this because we do not know how this medicine affects the unborn baby.
If you are a woman who can become pregnant, you must use contraception while you are being treated with this medicine and for at least 21 weeks after your last dose of Skyrizi.
If you are breastfeeding or plan to breastfeed, ask your doctor for advice before taking this medicine.
Driving and using machines
Skyrizi is unlikely to affect your ability to drive or use machines.
Skyrizi contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe, which is essentially “sodium-free”.
3. How to use Skyrizi
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, talk to your doctor or pharmacist again.
This medicine is given by injection under the skin (called a “subcutaneous injection”).
How much Skyrizi to use
Each dose is 150 mg given as a single injection. After the first dose, the next dose will be given 4 weeks later, and then every 12 weeks.
You and your doctor, pharmacist, or nurse will decide if you can inject this medicine yourself. You should not inject this medicine yourself unless your doctor, pharmacist, or nurse has taught you how to do it. It is also possible that the injection will be given by a caregiver who has learned how to do it.
Read section 7 “Instructions for use” at the end of this leaflet before giving yourself the Skyrizi injection.
If you use more Skyrizi than you should
If you have used more Skyrizi than you should or have given yourself a dose earlier than prescribed, talk to your doctor.
If you forget to use Skyrizi
If you forget to give yourself a dose of Skyrizi, you should give yourself a dose as soon as you remember. If you are unsure, talk to your doctor.
If you stop using Skyrizi
Do not stop using Skyrizi without talking to your doctor first. If you stop treatment, your symptoms may come back.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Talk to your doctor or seek medical attention right away if you have any symptoms of a serious infection, such as:
- fever, flu-like symptoms, night sweats
- feeling tired or having trouble breathing, persistent cough
- heat, redness, and pain in the skin or a painful skin rash with blisters
Your doctor will decide if you can continue using Skyrizi.
Other side effects
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects.
Very common:may affect more than 1 in 10 people
- upper respiratory tract infections with symptoms such as sore throat and nasal congestion
Common:may affect up to 1 in 10 people
- feeling tired
- fungal skin infections
- reactions at the injection site (such as redness or pain)
- itching
- headache
- rash
- eczema
Uncommon:may affect up to 1 in 100 people
- small red bumps on the skin
- hives (urticaria)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Skyrizi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the syringe and on the outer carton after EXP.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the pre-filled syringe in the original packaging to protect it from light.
If necessary, you can also store the pre-filled syringe at room temperature (up to 25°C) for a maximum of 24 hours in the original packaging to protect it from light.
Do not use this medicine if the liquid is cloudy or contains flakes or large particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Skyrizi Composition
- The active substance is risankizumab. Each prefilled syringe contains 150 mg of risankizumab in 1 ml of solution.
- The other ingredients are sodium acetate trihydrate, acetic acid, trehalose dihydrate, polysorbate 20, and water for injectable preparations.
Product Appearance and Container Contents
Skyrizi is a clear, colorless to yellow liquid contained in a prefilled syringe with a needle protector. The liquid may contain tiny, transparent or white particles.
EACH CONTAINER CONTAINS 1 PREFILLED SYRINGE
Marketing Authorization Holder
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Ludwigshafen
Germany
Manufacturer
AbbVie S.r.l.
04011 Campoverde di Aprilia
(Latina)
Italy
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien AbbVie SA Tel: +32 10 477811 | Lithuania AbbVie UAB Tel: +370 5 205 3023 |
| Luxembourg/Luxemburg AbbVie SA Belgique/Belgien Tel: +32 10 477811 |
Czech Republic AbbVie s.r.o. Tel: +420 233 098 111 | Hungary AbbVie Kft. Tel: +36 1 455 8600 |
Denmark AbbVie A/S Tlf: +45 72 30-20-28 | Malta V.J.Salomone Pharma Limited Tel: +356 22983201 |
Germany AbbVie Deutschland GmbH & Co. KG Tel: 00800 222843 33 (toll-free) Tel: +49 (0) 611 / 1720-0 | Netherlands AbbVie B.V. Tel: +31 (0)88 322 2843 |
Estonia AbbVie OÜ Tel: +372 623 1011 | Norway AbbVie AS Tlf: +47 67 81 80 00 |
Greece AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Τηλ: +30 214 4165 555 | Austria AbbVie GmbH Tel: +43 1 20589-0 |
Spain AbbVie Spain, S.L.U. Tel: +34 91 384 09 10 | Poland AbbVie Sp. z o.o. Tel: +48 22 372 78 00 |
France AbbVie Tél: +33 (0) 1 45 60 13 00 | Portugal AbbVie, Lda. Tel: +351 (0)21 1908400 |
Croatia AbbVie d.o.o. Tel: +385 (0)1 5625 501 | Romania AbbVie S.R.L. Tel: +40 21 529 30 35 |
Ireland AbbVie Limited Tel: +353 (0)1 4287900 | Slovenia AbbVie Biofarmacevtska družba d.o.o. Tel: +386 (1)32 08 060 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovak Republic AbbVie s.r.o. Tel: +421 2 5050 0777 |
Italy AbbVie S.r.l. Tel: +39 06 928921 | Finland AbbVie Oy Puh/Tel: +358 (0)10 2411 200 |
Cyprus Lifepharma (Z.A.M.) Ltd Τηλ: +357 22 34 74 40 | Sweden AbbVie AB Tel: +46 (0)8 684 44 600 |
Latvia AbbVie SIA Tel: +371 67605000 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Detailed and up-to-date information on this product is available below or on the outer packaging by scanning the QR code using a smartphone. The same information is also available on the following website:
www.skyrizi.eu
QR Code to be included
To request a copy of this leaflet in
- Instructions for Use
Read the entire section 7 before using Skyrizi
Skyrizi Prefilled Syringe
Plunger Wings of restraint Needle cap
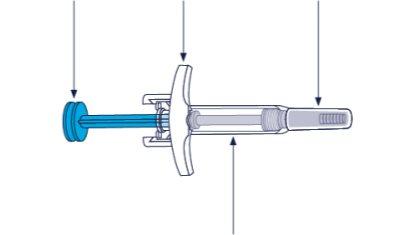
Syringe Body
Important Information You Should Know Before Injecting Skyrizi
- You must have received training on how to inject Skyrizi before administering an injection. If you need help, consult your doctor, pharmacist, or nurse.
- Mark the dates on a calendar to know when it's time to inject Skyrizi.
- Keep Skyrizi in its original packaging to protect the medication from light until the time of use.
- Do notinject the medication if the liquid is cloudy or contains flakes or large particles. The liquid should be clear to yellow and may contain tiny, transparent or white particles.
- Do notshake the syringe.
- Wait to remove the needle cap until the moment before injection.
Return this medication to the pharmacy
- after the expiration date (EXP) indicated.
- if the liquid has been frozen at any time (even if it has been thawed).
- if the syringe has been dropped or damaged.
- if the perforations on the box are broken.
For a more comfortable injection: Remove the box from the refrigerator and let it sit at room temperature, away from direct sunlight, for 15 to 30minutesbefore injection.
- Skyrizi should not be heated in any other way (e.g., in a microwave or hot water).
- Keep the syringe in the box until the time of injection.
Follow these steps each time you use Skyrizi
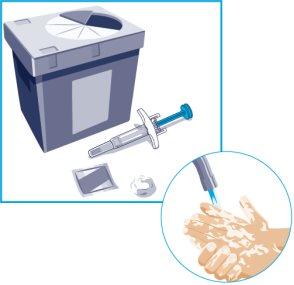
STEP 1
| Remove the prefilled syringe from the carton by holding the wings of restraint.
On a flat, clean surface, place the following:
Wash and dry your hands. |
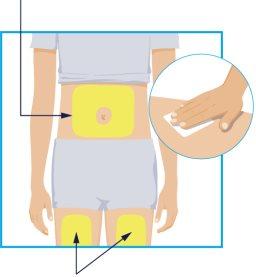
STEP 2 Injection Sites Injection Sites | Choose one of these 3 areas for injection:
Before injection, clean the injection site with an alcohol swab using a circular motion.
|
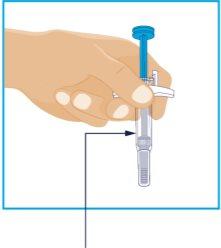
STEP 3
Inspect the Liquid | Hold the syringe with the needle covered by the needle cap facing down as shown. Check the liquid in the syringe.
|
STEP 4
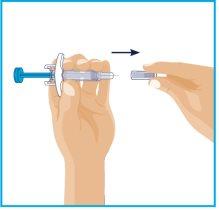
| Remove the needle cap:
Do nottouch the needle with your fingers or let it come into contact with anything. |
STEP 5
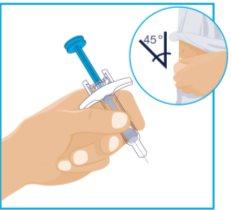
| Hold the syringe body with one hand between the thumb and index finger, as if holding a pencil. Gently pinch the cleaned skin with the other hand and hold firmly. Insert the needle into the skin at an approximate 45-degree angle, with a short and quick motion. Keep the syringe steady at the same angle. |
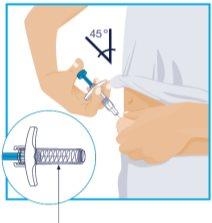
STEP 6
Needle Protector | Slowly push the plunger until it reaches the end, until all the liquid has been injected. Slowly remove the needle from the skin, keeping the syringe at the same angle. Slowly release your thumb from the plunger. The needle will then be covered by the needle protector.
Press a cotton ball or gauze over the injection site and hold for 10 seconds. Do notrub the skin at the injection site. There may be slight bleeding at the injection site. This is normal. |
STEP 7
| Discard the used syringe in a sharps container immediately after use.
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SKYRIZI 150 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 150 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: INJECTABLE, 180 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: INJECTABLE, 360 mgActive substance: risankizumabManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription required
Online doctors for SKYRIZI 150 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about SKYRIZI 150 mg PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions