SIBUDEL 250 mg Injectable Solution in Pre-filled Syringe
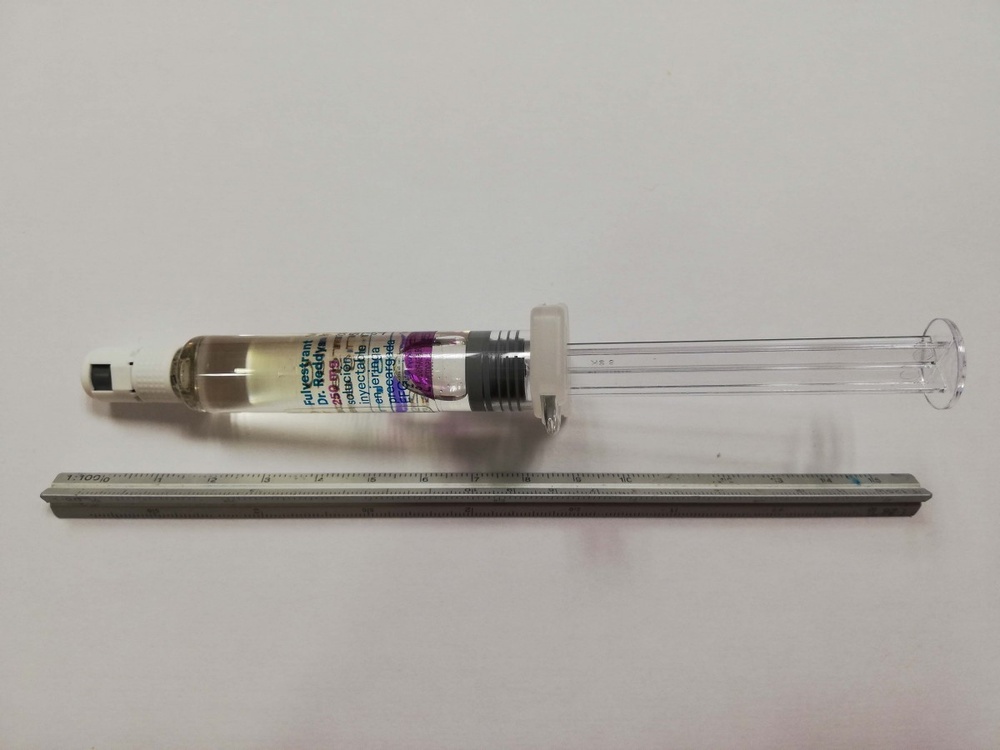

How to use SIBUDEL 250 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet:information for the user
Sibudel 250 mg solution for injection in a pre-filled syringe EFG
fulvestrant
Read all of this leaflet carefully before you start using this medicine,because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only, and you should not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Sibudel and what is it used for
- What you need to know before you use Sibudel
- How to use Sibudel
- Possible side effects
- Storage of Sibudel
- Contents of the pack and further information
1. What is Sibudel and what is it used for
Sibudel contains the active substance fulvestrant, which belongs to the group of estrogen blockers. Estrogens, a type of female sex hormone, may be involved in the development of breast cancer in some cases.
Sibudel is used:
- alone, to treat postmenopausal women with a type of breast cancer called hormone receptor-positive breast cancer, which is locally advanced or has spread to other parts of the body (metastatic) or,
- in combination with palbociclib to treat women with a type of breast cancer called hormone receptor-positive, human epidermal growth factor receptor 2 (HER2) negative breast cancer, which is locally advanced or has spread to other parts of the body (metastatic). Women who have not reached menopause will also be treated with a medicine called a luteinizing hormone-releasing hormone (LHRH) agonist.
Fulvestrant may be administered in combination with palbociclib. It is important that you also read the package leaflet for palbociclib. If you have any questions about palbociclib, ask your doctor.
2. What you need to know before you use Sibudel
Do not useSibudel:
- if you are allergic to fulvestrant or any of the other ingredients of this medicine (listed in section 6)
- if you are pregnant or breastfeeding (see section "Pregnancy and breastfeeding")
- if you have severe liver problems
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting Sibudel if any of the following apply to you:
- kidney or liver problems
- low platelet count (which helps blood to clot) or bleeding disorders
- previous blood clot problems
- osteoporosis (loss of bone density)
- alcoholism (see section "Sibudel contains ethanol 96% (alcohol)").
The efficacy and safety of fulvestrant (as monotherapy or in combination with palbociclib) have not been studied in patients with critical visceral disease.
Children and adolescents
Sibudel is not indicated for children and adolescents under 18 years of age.
Using Sibudelwithother medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, you should tell your doctor if you are using anticoagulants (medicines to prevent blood clots).
Pregnancy andbreastfeeding
You should not use Sibudel if you are pregnant. If you can become pregnant, you should use an effective contraceptive method while being treated with Sibudel and for 2 years after your last dose.
You should not breastfeed while being treated with Sibudel.
Driving and using machines
Sibudel is not expected to affect your ability to drive or use machines. However, if you feel tired after treatment, do not drive or use machines.
Sibudelcontains ethanol 96% (alcohol)
This medicine contains 474 mg of alcohol (ethanol) in each 5 ml pre-filled syringe, which corresponds to 94.8 mg/ml. The amount in a 10 ml dose of this medicine is equivalent to less than 24 ml of beer or 10 ml of wine.
The small amount of alcohol in this medicine will not have a notable effect.
Sibudelcontains benzyl alcohol
This medicine contains 500 mg of benzyl alcohol in each 5 ml pre-filled syringe, equivalent to 100 mg per ml.
Benzyl alcohol may cause allergic reactions.
Talk to your doctor or pharmacist if you have liver or kidney disease. This is because large amounts of benzyl alcohol can accumulate in the body and cause side effects (metabolic acidosis).
Sibudelcontains benzyl benzoate
This medicine contains 750 mg of benzyl benzoate in each 5 ml pre-filled syringe, equivalent to 150 mg per ml.
3. How to use Sibudel
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is 500 mg of fulvestrant (two 250 mg/5ml injections) administered once a month with an additional dose of 500 mg administered 2 weeks after the initial dose.
Your doctor or nurse will administer Sibudel by slow intramuscular injection into each of your buttocks.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You may need urgent medical attention if you experience any of the following side effects:
- allergic reactions (hypersensitivity), including swelling of the face, lips, tongue, and/or throat, which may be signs of anaphylactic reactions
- thromboembolism (increased risk of blood clots)*
- liver inflammation (hepatitis)
- liver failure.
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects:
Side effects reported in patients treated with fulvestrant as monotherapy:
Very common:may affect more than 1 in 10 people
- injection site reactions, such as pain and/or inflammation
- abnormal liver enzyme levels (in blood tests)*
- nausea (feeling sick)
- weakness, fatigue*
- joint and musculoskeletal pain
- hot flashes
- skin rash
- allergic reactions (hypersensitivity), including swelling of the face, lips, tongue, and/or throat
All other side effects:
Common:may affect up to 1 in 10 people
- headache
- vomiting, diarrhea, or loss of appetite*
- urinary tract infections
- back pain*
- increased bilirubin levels (a bile pigment produced by the liver).
- thromboembolism (increased risk of blood clots)*
- decreased platelet count (thrombocytopenia)
- vaginal bleeding
- lower back pain radiating to one leg (sciatica)
- sudden weakness, numbness, tingling, or loss of movement in your leg, especially on one side of the body, sudden problems with walking or balance (peripheral neuropathy)
Uncommon:may affect up to 1 in 100 people
- thick, white, vaginal discharge and candidiasis (infection)
- hematoma and bleeding at the injection site
- increased gamma-GT, a liver enzyme identified in a blood test
- liver inflammation (hepatitis)
- liver failure
- numbness, tingling, and pain
- anaphylactic reactions
*Includes side effects for which the exact role of Sibudel cannot be evaluated due to the underlying disease.
Side effects reported in patients treated with fulvestrant in combination with palbociclib:
Very common:may affect more than 1 in 10 people
- decreased neutrophil count (neutropenia)
- decreased white blood cell count (leucopenia)
- infections
- fatigue
- nausea
- reduced red blood cell count (anemia)
- inflammation or ulceration of the mouth
- diarrhea
- decreased platelet count (thrombocytopenia)
- vomiting
- hair loss
- rash
- loss of appetite
- fever
Common:may affect up to 1 in 10 people
- feeling weak
- increased liver enzyme levels
- loss of taste
- nasal bleeding
- excessive tearing
- dry skin
- blurred vision
- dry eyes
Uncommon:may affect up to 1 in 100 people
fever with other signs of infection (febrile neutropenia).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Sibudel
Keepthis medicineout of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton or on the label of the syringes after EXP. The expiry date refers to the last day of the month shown.
Do not use this medicine if you notice particles or discoloration before administration.
Store and transport in a refrigerator (between 2°C and 8°C).
Temperature deviations outside the range of 2°C to 8°C should be controlled. This includes avoiding storage at temperatures above 25°C, and not exceeding a period of 4 months, during which the average storage temperature of the medicine is below 25°C (but above 2°C to 8°C). After temperature deviations, the medicine should be returned immediately to the recommended storage conditions (store and transport in a refrigerator between 2°C and 8°C). Temperature deviations have a cumulative effect on the quality of the medicine, and the 4-month period should not exceed the 2-year shelf life of Sibudel. Exposure to temperatures below 2°C will not damage the medicine, as long as it is not stored below -20°C.
Keep the pre-filled syringe in the original packaging to protect it from light.
Your healthcare professional will be responsible for the proper storage, use, and disposal of Sibudel.
This medicine may pose a risk to the aquatic environment. Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the SIGRE collection point at your pharmacy. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Sibudel
- The active substance is fulvestrant. Each pre-filled syringe contains 250 mg of fulvestrant. Each ml of solution contains 50 mg of fulvestrant.
- The other ingredients (excipients) are ethanol (96%), benzyl alcohol, benzyl benzoate, and refined castor oil.
Appearance of the product and pack contents
Sibudel is a viscous, clear, colorless to yellow solution in a pre-filled syringe equipped with a Luer-Lock connector containing 5 ml of injectable solution. Two syringes should be administered to receive the recommended monthly dose of 500 mg.
Sibudel is available in 2 formats:
- 1 pack containing 1 glass pre-filled syringe and 1 needle with a safety system for connection to the syringe body.
- 1 pack containing 2 glass pre-filled syringes and 2 needles with a safety system for connection to the syringe body.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Teva Pharma, S.L.U.
C/ Anabel Segura, 11. Edificio Albatros B, 1ª planta
28108 Alcobendas, Madrid
Spain
Manufacturer
Pliva Croatia Ltd.
Prilaz baruna Filipovica 25
10000 Zagreb
Croatia
This medicine is authorised in the Member States of the European Economic Area under the following names:
Germany: Fulvestrant AbZ 250 mg Injektionslösung in einer Fertigspritze
Spain: Sibudel 250 mg solución inyectable en jeringa precargada EFG
Date of last revision of thisleaflet: July 2025
Other sources of information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) https://www.aemps.gob.es/
You can access detailed and up-to-date information about this medicine by scanning the QR code included in the packaging with your smartphone. You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/80999/P_80999.html
QR code + URL
----------------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Sibudel 500 mg (2 x 250 mg/5 ml solution for injection) should be administered using two pre-filled syringes (see section 3).
Administration instructions
Administer the injection according to local guidelines for intramuscular injection of large volumes.
NOTE: Due to the proximity of the sciatic nerve, caution should be exercised when injecting Sibudel into the dorsogluteal area (see section 4.4).
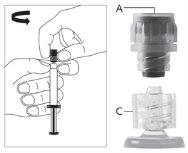
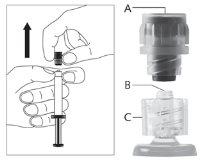
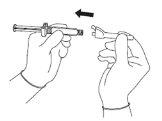
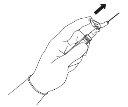
Warning - Do not sterilize the needle with a safety system in an autoclave before use. Hands should remain behind the needle at all times during use and disposal.
For each of the two syringes:
| Figure 1
|
| Figure 2
|
| Figure 3
|
| Figure 4
|
NOTE: Activate away from your body and others. Listen for the click and visually confirm that the needle tip is fully protected. | Figure 5
|
Disposal
The pre-filled syringes are for singleuse only.
This medicine may pose a risk to the aquatic environment. Disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Average pharmacy price225.7 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SIBUDEL 250 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 250 mg/5 mlActive substance: fulvestrantManufacturer: Bexal Farmaceutica S.A.Prescription requiredDosage form: INJECTABLE, 250 mgActive substance: fulvestrantManufacturer: Ever Valinject GmbhPrescription requiredDosage form: INJECTABLE, 250 mgActive substance: fulvestrantManufacturer: Astrazeneca AbPrescription required
Online doctors for SIBUDEL 250 mg Injectable Solution in Pre-filled Syringe
Discuss questions about SIBUDEL 250 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions