
RYALTRIS 25 micrograms/600 micrograms/per actuation nasal spray suspension

How to use RYALTRIS 25 micrograms/600 micrograms/per actuation nasal spray suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ryaltris 25 micrograms/600 micrograms per actuation, nasal spray suspension
mometasone furoate/olopatadine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Ryaltris is and what it is used for
- What you need to know before you use Ryaltris
- How to use Ryaltris
- Possible side effects
- Storing Ryaltris
- Contents of the pack and other information
1. What Ryaltris is and what it is used for
Ryaltris contains two active substances: mometasone furoate and olopatadine.
- Mometasone furoate belongs to a group of medicines called corticosteroids (steroids) that reduce inflammation, which often occurs in allergic rhinitis.
- Olopatadine belongs to a group of medicines called antihistamines. Antihistamines work by preventing the effects of substances like histamine that the body produces as part of an allergic reaction, thus reducing the symptoms of allergic rhinitis.
Ryaltris is used to treat the symptoms of moderate to severe seasonal allergic rhinitis(also known as hay fever) and perennial allergic rhinitisin adults and adolescents 12 years and older.
Seasonal allergic rhinitis(hay fever) is an allergic reaction that occurs at certain times of the year and is caused by inhaling pollen from trees, grasses, weeds, and also molds and fungal spores.
Perennial allergic rhinitisoccurs throughout the year and symptoms can be caused by sensitivity to a variety of things including house dust mites, animal hair (or dander), feathers, and certain foods.
Ryaltris relieves the symptoms of allergies, such as nasal discharge, sneezing, itching, and nasal congestion.
2. What you need to know before you use Ryaltris
Do not use Ryaltris
- if you are allergicto mometasone furoate, olopatadine, or any of the other ingredients of this medicine (listed in section 6).
- if you have an untreated nasal infection. Using Ryaltris while you have an untreated nasal infection, such as herpes, can make the infection worse. You should wait until the infection has cleared up before starting to use the nasal spray.
- if you have had an operationor a recent injuryto your nose. You should not use the nasal spray until your nose has healed.
Warnings and precautions
Consult your doctor or pharmacist before you start using Ryaltris
- if you have or have ever had tuberculosis.
- if you have any other infection.
- if you are taking other corticosteroids, either by mouth or by injection.
Consult your doctor or pharmacist while you are using Ryaltris
- if you have trouble fighting off infections (your immune system is not working well) and you come into contact with someone who has measles or chickenpox. You should avoid contact with anyone who has these infections.
- if you have a nose or throat infection.
- if you have been using this medicine for several monthsor longer.
- if you have persistent irritation of your nose or throat.
- if you have blurred visionor other changes in your vision.
If nasal corticosteroid sprays are used at high doses for long periods of time, side effects can occur due to the medicine being absorbed into the body. These side effects can include weight loss, fatigue, muscle weakness, low blood sugar levels, low sodium levels, joint pain, depression, and skin darkening. If you experience any of these side effects, your doctor may recommend a different medicine during periods of stress or scheduled surgeries.
If you are not sure if the above applies to you, consult your doctor or pharmacist before using Ryaltris.
Children and adolescents
Ryaltris is not recommended for children under 12 years of age.
Using Ryaltris for a long period of time may cause children and adolescents to grow more slowly. Your doctor will regularly check your child's heightand make sure they are taking the lowest effective dose possible.
Other medicines and Ryaltris
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you are taking other corticosteroids for your allergy, either by mouth or by injection, your doctor may advise you to stop taking them when you start using Ryaltris.
If you are taking other medicines by mouth or using them locally (eye drops or nasal sprays) that contain olopatadine or other antihistamines, your doctor may recommend that you stop taking them once you start using Ryaltris.
Some medicines may increase the effects of Ryaltris, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Ryaltris should not be used during pregnancy unless your doctor considers it necessary.
If you are using Ryaltris, your doctor will confirm with you whether you should breastfeed your baby, taking into account the benefit to you of the treatment and the benefit of breastfeeding your baby. You should not do both.
Driving and using machines
Very rarely, you may experience dizziness, drowsiness, fatigue, and sleepiness. If this happens, do not drive or use machinery. Note that drinking alcohol can enhance these effects.
Ryaltris contains benzalkonium chloride
This medicine contains 0.02 mg of benzalkonium chloride in each nasal spray.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for long periods of treatment.
3. How to use Ryaltris
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, consult your doctor or pharmacist again.
Avoid contact with the eyes.
Adults and adolescents (12 years of age and older)
The recommended dose is two sprays in each nostrilin the morning and in the evening.
Use in children under 12 years of age
This medicine is not recommended for children under 12 years of age.
Method of administration
The spray is for nasal use.
Read the following instructions carefully and use the spray only as directed.
Shake the bottle for at least 10 seconds before each use.
When Ryaltris is not in use, the purple cap should always be kept firmly on the white tip of the nozzle.
Ryaltris nasal spray bottle
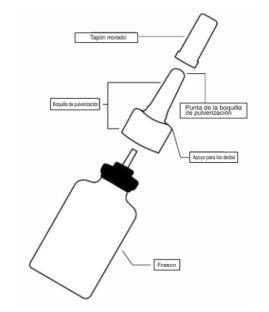
Preparing the bottle for nasal spray
- Shake the bottle for at least 10 seconds and then remove the purple cap (see figure 1).
- If you are using the spray for the first time, you must "prime" the bottle by pumping it into the air.
- Hold the nasal spray firmly and in an upright position with your index and middle fingers on either side of the spray nozzle (on the finger rests) while holding the ridged base of the bottle with your thumb.
- Point the nozzle away from you and then press down and release the pump 6 times until a fine mist appears (see figure 2).
- Now your pump is primed and ready to use.
- If you have not used the spray for 14 days or more, you must shake the bottle well and "re-prime" it by pumping the spray 2 times or until a fine mist appears.
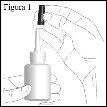
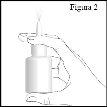
How to use the nasal spray
- Shake the bottle for at least 10 seconds before each use (morning and evening).
- Gently blow your nose to clear your nostrils.
- Hold the bottle firmly with your index and middle fingers on either side of the spray nozzle (on the finger rests) while holding the ridged base of the bottle with your thumb.
- Close one nostril with your finger and carefully insert the tip of the spray nozzle into the other nostril, pointing it slightly outward (see figure 3).
- Lean your head slightly forward. Press the finger rests quickly and firmly to activate the pump.
- Breathe in gently through your nose while spraying. Then breathe out through your mouth (see figure 4).
- Repeat the above steps and apply a second spray to the same nostril.
- Repeat with 2 sprays in the other nostril.
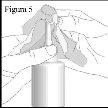
- To avoid any blockage, after each use, carefully clean the nozzle with a tissue or a clean, dry cloth (see figure 5).
- Hold the nozzle and push the purple cap towards the base, on the nozzle, until you hear a click (see figure 6).



Cleaning your nasal spray
If the nozzle becomes blocked, follow the steps below:
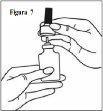
- Gently pull the spray nozzle upwards (see figure 7). Remove the purple cap and place only the nozzle in warm water to soak it.
- Do not try to unblock the nasal spray by inserting a pin or other pointed object, as this will damage the spray and cause you to not receive the correct dose of medicine.
- After soaking the nozzle for 15 minutes, rinse the nozzle and the purple cap with warm water and let them dry completely.
- Put the purple cap back on the nozzle and put it back on the bottle.
- After following the steps to clean the blocked nozzle, refer to the previous section "Preparing the bottle for nasal spray" and re-prime it using 2 sprays. Put the purple cap back on and your Ryaltris will be ready to use.
- Repeat the unblocking steps if necessary.
If you use more Ryaltris than you should
It is unlikely that you will have any problems, but if you are concerned or if you have used higher doses than recommended for a long time, consult your doctor.
If you use steroids for a long time or in large amounts, they can, in rare cases, affect some of your hormones. In children, it can affect growth and development.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, stating the medicine and the amount taken.
If you forget to use Ryaltris
If you forget to use your nasal spray at the right time, use it as soon as you remember, then follow your normal routine of administration. Do not use a double dose to make up for forgotten doses.
If you stop using Ryaltris
It is very important that you use your nasal spray regularly. Do not stop your treatment even if you feel better, unless your doctor tells you to.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Immediate allergic reactions (hypersensitivity) can occur after using this medicine. These reactions can be serious. You should stop using Ryaltris and seek medical help immediately if you experience symptoms such as: swollen face, tongue, or pharynx, difficulty swallowing, hives, fatigue, or difficulty breathing.
Common (may affect up to 1 in 10 people):
- A bitter taste in the mouth;
- Nasal bleeding;
- Mild irritation of the inside of the nose;
Uncommon (may affect up to 1 in 100 people):
- Dizziness;
- Headaches;
- Drowsiness;
- Nasal dryness;
- Dry mouth;
- Abdominal pain;
- Nausea;
- Fatigue;
Rare (may affect up to 1 in 1,000 people):
- Bacterial vaginosis (bacterial infection of the vagina);
- Anxiety, depression, insomnia;
- Lethargy, migraine;
- Dry eyes, blurred vision, eye discomfort;
- Ear pain;
- Sore throat;
- Sneezing;
- Throat irritation;
- Constipation;
- Tongue pain;
- Swelling and ulcers inside the nose.
Frequency not known (frequency cannot be estimated from the available data):
- Increased eye pressure (glaucoma) and/or cataracts causing vision changes;
- Damage to the nasal septum that separates the nostrils;
- Difficulty breathing and/or wheezing;
- Respiratory tract infection.
Systemic side effects (side effects that affect the whole body) can occur when this medicine is used at high doses for a long time. It is much less likely that these effects will occur if you use a nasal steroid spray than if you take steroids by mouth. These effects can vary depending on the patient.
Nasal steroids can affect the normal production of hormones in your body, particularly if you use high doses for a long time. In children and adolescents, this side effect can cause them to grow more slowly than others.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Ryaltris
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and carton after "EXP". The expiry date is the last day of the month stated. Do not freeze. The spray should be used within 2 months of first use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container contents and additional information
Ryaltris composition
- The active ingredients are: mometasone furoate (as monohydrate) and olopatadine (as hydrochloride). Each released dose (dose that comes out of the spray) contains mometasone furoate monohydrate equivalent to 25 micrograms of mometasone furoate and olopatadine hydrochloride equivalent to 600 micrograms of olopatadine.
- The other components are: microcrystalline cellulose (E 460), sodium carmellose (E 466), sodium phosphate dibasic heptahydrate (E 339), sodium chloride, benzalkonium chloride, glycerol, disodium edetate, polysorbate 80 (E 433), hydrochloric acid (E 507), sodium hydroxide (E 524), and water for injectable preparations.
Product appearance and container contents
Ryaltris is a white and homogeneous suspension.
Ryaltris is presented in a white high-density polyethylene bottle with a manual polypropylene spray pump, with a calibrated dose. The nasal applicator is equipped with a violet PEAD cap.
Container sizes:
1 bottle of 20 ml with 56 sprays
1 bottle of 20 ml with 120 sprays
1 bottle of 30 ml with 240 sprays
Only some container sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Glenmark Pharmaceuticals s.r.o.
Hvezdova 1716/2b
140 78 Praha 4
Czech Republic
Manufacturer
Glenmark Pharmaceuticals s.r.o.
Fibichova 143, 566 17 Vysoke Myto
Czech Republic
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Laboratorios Menarini, S.A.
Alfons XII, 587
08918 Badalona (Barcelona)
Spain
This medicine is authorized in the EEA member states under the following names:
Germany | Ryaltris 25 micrograms/600 micrograms per spray Nasal Spray, Suspension. |
Austria | RYALTRIS 25 micrograms/600 micrograms per spray Nasal Spray, Suspension |
Belgium | RYALTRIS 25 micrograms/spray + 600 micrograms/spray, suspension for nasal spray RYALTRIS 25 microgram/spray + 600 microgram/spray, nasal spray, suspension RYALTRIS 25 micrograms/spray + 600 micrograms/spray Nasal Spray, Suspension |
Denmark | Ryaltris 25 micrograms/600 micrograms per dose Nasal Spray, suspension |
Slovakia | RYALTRIS 25 micrograms/600 micrograms in one spray nasal suspension aerosol |
Spain | Ryaltris 25 micrograms/600 micrograms/pulse, suspension for nasal spray |
Finland | RYALTRIS 25 micrograms + 600 micrograms / dose nasal spray, suspension |
France | RYALTRIS 25 micrograms/600 micrograms, suspension for nasal spray |
Ireland | Ryaltris 25 micrograms/actuation + 600 micrograms/actuation nasal spray, suspension |
Italy | RYALTRIS 25 micrograms/600 micrograms per delivery Nasal Spray, suspension |
Norway | RYALTRIS |
Netherlands | RYALTRIS 25 micrograms/600 micrograms, nasal spray, suspension |
Poland | RYALTRIS |
United Kingdom | RYALTRIS 25 micrograms/600 micrograms per actuation Nasal Spray, suspension |
Czech Republic | RYALTRIS |
Romania | RYALTRIS 25 micrograms/600 micrograms/dose nasal spray suspension |
Sweden | Ryaltris, 25 micrograms/puff + 600 micrograms/puff, Nasal Spray, suspension |
Date of the last revision of this leaflet:July 2024.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Average pharmacy price14.75 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RYALTRIS 25 micrograms/600 micrograms/per actuation nasal spray suspensionDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: NASAL PRODUCT, 27.5 MICROGRAMS/SPRAYActive substance: fluticasone furoateManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: NASAL PRODUCT, 137 micrograms/50 micrograms/applicationActive substance: fluticasone, combinationsManufacturer: Laboratorios Cinfa S.A.Prescription required
Online doctors for RYALTRIS 25 micrograms/600 micrograms/per actuation nasal spray suspension
Discuss questions about RYALTRIS 25 micrograms/600 micrograms/per actuation nasal spray suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











