
Rixubis 250 IU/vial powder and solvent for injectable solution

How to use Rixubis 250 IU/vial powder and solvent for injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
RIXUBIS250UI Powder andsolvent for solution for injection
RIXUBIS500UI Powder andsolvent for solution for injection
RIXUBIS1000UI Powder andsolvent for solution for injection
RIXUBIS2000UI Powder andsolvent for solution for injection
RIXUBIS3000UI Powder andsolvent for solution for injection
Nonacog gamma (recombinant human coagulation factor IX)
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the package leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is RIXUBIS and what is it used for
- What you need to know before you use RIXUBIS
- How to use RIXUBIS
- Possible side effects
- Storage of RIXUBIS
- Contents of the pack and other information
1. What is RIXUBIS and what is it used for
RIXUBIS contains the active substance nonacog gamma and is a human coagulation factor IX. Factor IX is a normal component of human blood necessary for its proper coagulation. RIXUBIS is used in patients with hemophilia B (Christmas disease, a hereditary blood disorder caused by a deficiency of factor IX). It acts by replacing the missing factor IX to allow the patient's blood to clot.
RIXUBIS is used for the treatment and prevention of bleeding in patients with hemophilia B of all age groups.
2. What you need to know before you use RIXUBIS
Do not use RIXUBIS
- if you are allergic to nonacog gamma or any of the other ingredients of this medicine (listed in section 6)
- if you are allergic to hamster proteins
Warnings andprecautions
It is possible that allergic-type hypersensitivity reactions may occur with RIXUBIS. Stop the infusion and contact your doctor immediately or seek urgent medical attention if you experience the first signs of hypersensitivity reactions/allergy such as hives, rash, chest tightness, wheezing, low blood pressure, or anaphylaxis (a severe allergic reaction that can cause difficulty swallowing and/or breathing, red and/or swollen face and/or hands). Your doctor may need to treat you immediately in case of these reactions. Your doctor may also perform a blood test to check if you have developed neutralizing antibodies (inhibitors) against the medicine, as inhibitors may develop along with allergies. Patients with factor IX inhibitors may have a higher risk of anaphylaxis during subsequent treatment with factor IX.
Consult your doctor immediately if the bleeding does not stop as expected or if you experience a significant increase in the use of RIXUBIS to control a bleed. Your doctor will perform a blood test to check if you have developed neutralizing antibodies (inhibitors) against RIXUBIS. The risk of developing inhibitors is higher in patients who have not been previously treated with a factor IX substitute medicine or in the early stages of treatment, i.e., in young children.
The production of factor IX in the body is controlled by the factor IX gene. Patients who have specific mutations in their factor IX gene, such as a large deletion, may be more likely to have factor IX inhibitors and an allergic reaction in the early stages with any factor IX concentrate. Therefore, if you are known to have such a mutation, your doctor will monitor you more closely for signs of an allergic reaction.
If you have liver or heart disease, or if you have recently undergone major surgery, inform your doctor, as there is a higher risk of complications in blood coagulation.
There have been reports of kidney disorders (nephrotic syndrome) after administration of high doses of factor IX in patients with hemophilia B who had factor IX inhibitors and a history of allergic reactions.
Whenever possible, record the name of the medicine and the batch number each time you use RIXUBIS (e.g., in your diary) to keep a record of the medicines and batches you have used.
Using RIXUBIS with other medicines
Tell your doctor if you are using, have recently used, or might use any other medicines. No interactions of RIXUBIS with other medicines have been reported.
Pregnancy, breastfeeding, andfertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine. Hemophilia B occurs very rarely in women.
Driving andusing machines
RIXUBIS has no influence on the ability to drive and use machines.
RIXUBIS contains sodium
This medicine contains 19 mg of sodium per vial, which should be taken into consideration in patients on a low-sodium diet.
3. How to use RIXUBIS
Treatment with RIXUBIS will be started by a doctor experienced in the treatment of patients with hemophilia B.
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor again.
Your doctor will decide the dose of RIXUBIS that you will be given. This dose and the duration will depend on the severity of your factor IX deficiency, the location and extent of the bleeding, as well as your clinical condition, age, and the rate at which your body consumes factor IX, which should be checked regularly.
Your doctor or nurse will administer RIXUBIS to you by intravenous (IV) infusion after reconstituting the powder with the supplied solvent. You or another person can also administer the injection of RIXUBIS, but only after receiving proper training.
Reconstitution and administration
- For reconstitution, use only the solvent and the reconstitution device (BAXJECT II) provided in the package.
- For administration, a luer lock syringe is required.
- Do not use if the BAXJECT II equipment, the sterile protector, or its packaging is damaged or shows signs of deterioration.
Reconstitution
Use aseptic technique:
- If the medicine is in the refrigerator, remove the RIXUBIS powder and solvent vials from the refrigerator and let them reach room temperature (between 15°C and 30°C).
- Wash your hands with soap and warm water.
- Remove the protectors from the powder and solvent vials.
- Clean the stoppers with the alcohol-impregnated wipes. Place the vials on a flat and clean surface.
- Open the BaxJect II equipment package by removing the paper cover without touching the inside (Fig. a). Do not remove the equipment from the package.
- Turn the package over and insert the plastic tip through the solvent stopper. Hold the package by its end and remove the BaxJect II equipment from its package (Fig. b). Do not remove the blue protector from the BAXJECT II equipment.
- With BaxJect II attached to the solvent vial, turn the system over so that the solvent vial is on top of the equipment. Insert the white plastic tip into the RIXUBIS powder vial stopper. The vacuum will draw the solvent into the RIXUBIS powder vial (Fig. c).
- Gently swirl until all the material is dissolved. The medicine dissolves quickly (in about 2 minutes). Make sure that RIXUBIS is completely dissolved; if not, the reconstituted solution will not pass through the filter of the equipment. The reconstituted medicine should be inspected visually for particles or discoloration before administration. The solution should be clear or slightly opalescent. Do not use cloudy or precipitated solutions.
Fig. aFig. bFig. c



Do not refrigerate the preparation after reconstitution.
Use immediately.
Administration
Use aseptic technique:
- Remove the blue protector from the BAXJECT II equipment. Do not introduce air into the syringe. Connect the syringe to the BAXJECT II equipment (Fig. d).
- Turn the system over (the vial with the reconstituted solution on top). Draw the reconstituted solution into the syringe by slowly pulling the plunger back (Fig. e).
- Disconnect the syringe.
- Connect a winged infusion needle to the syringe. Inject intravenously. The solution should be administered slowly, at a rate determined by the patient's comfort level, not exceeding 10 ml per minute.
Fig. dFig. e
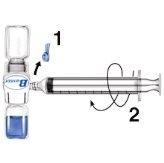
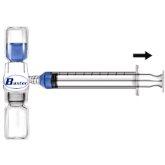
Whenever possible, record the name of the medicine and the batch number each time you use RIXUBIS (e.g., in your diary) to keep a record of the medicines and batches you have used.
The disposal of unused medicine and all materials that have been in contact with it will be done according to local regulations.
If you use more RIXUBIS than you should
Follow the instructions for administration of RIXUBIS exactly as told by your doctor. If you are unsure, consult your doctor again. If you inject a higher dose of RIXUBIS than recommended, consult your doctor as soon as possible.
If you forget to use RIXUBIS
Do not inject a double dose to make up for forgotten doses. Administer the next injection as scheduled and continue as instructed by your doctor.
If you stop using RIXUBIS
Do not stop using RIXUBIS without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
It is possible that allergic-type hypersensitivity reactions may occur with RIXUBIS. These reactions include burning and stinging at the infusion site, chills, flushing, lethargy, restlessness, tingling, hives, itching, and rash, low blood pressure, rapid heart rate, chest tightness, wheezing, throat swelling, anaphylaxis (a severe allergic reaction), headache, nausea, and vomiting. Consult your doctor immediately if you experience these signs. Your doctor may need to treat you immediately in case of these reactions (see section 2 'Warnings and precautions').
The following side effects have been observed with RIXUBIS:
Common side effects(may affect up to 1 in 10 people)
- altered taste
- pain in the limbs
Uncommon side effects(frequency cannot be estimated from the available data)
- allergic reactions (hypersensitivity)
No problems caused by excessive blood coagulation (thromboembolic events) have been observed with this medicine, but they can occur with any factor IX product. These include heart attacks, blood clots in the veins or lungs.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of RIXUBIS
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging and the vial label after EXP. The expiry date is the last day of the month stated.
Store below 30°C.
Do not freeze.
Use the reconstituted solution immediately.
Do not use RIXUBIS if the solution is not colorless and transparent.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
RIXUBIS Composition
- The active ingredient is nonacog gamma (recombinant human coagulation factor IX). Each vial contains nominally 250, 500, 1000, 2000, or 3000 IU, corresponding to a concentration of 50, 100, 200, 400, or 600 IU/ml after reconstitution with 5 ml of solvent.
- The other components of the powder are sucrose, mannitol, sodium chloride, calcium chloride, L-histidine, and polysorbate 80.
Solvent vial: 5 ml of sterile water for injectable preparations.
Product Appearance andContainer Contents
RIXUBIS is provided as a powder and solvent for solution for injection.
The container contents are as follows:
- one vial of RIXUBIS powder 250, 500, 1000, 2000, or 3000 IU in a glass vial with a rubber stopper
- one vial of 5 ml of sterile water for injectable preparations in a glass vial with a rubber stopper
- one BAXJECT II (needleless reconstitution device)
Marketing Authorization Holder
Baxalta Innovations GmbH
Industriestrasse 67
A-1221 Vienna
Manufacturer
Baxalta Belgium Manufacturing SA
Boulevard René Branquart 80
B-7860 Lessines
Belgium
Baxter SA
Boulevard René Branquart 80
B-7860 Lessines
Belgium
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
België/Belgique/Belgien Baxalta Belgium SPRL Tel: +32 2 892 62 00 | Lietuva UAB "Baxter Lithuania" Tel: +370 5 269 16 90/+370 5 252 71 00 |
България Бакстер България ЕООД Тел.: +359 2 9808482 | Luxembourg/Luxemburg Baxalta Belgium SPRL Tel: +32 2 892 62 00 |
Česká republika Baxter Czech spol.s.r.o. Tel.: +420 225774111 | Magyarország Baxter Hungary Kft. Tel.: +361 202 19 80 |
Danmark Baxalta Denmark A/S Tlf: +45 32 70 12 00 | Malta Baxalta UK Limited Tel.: +44 1 635 798 777 |
Deutschland Baxalta Deutschland GmbH Tel: +49 89 262077-011 | Nederland Baxalta Netherlands B.V. Tel: +31 30 799 27 77 |
Eesti OÜ Baxter Estonia Tel.: +372 6 515 120 | Norge Baxalta AS Tlf: +47 22 585 000 |
Ελλάδα Baxter (Hellas) Ε.Π.Ε. Τηλ.: +30 210 28 80 000 | Österreich Baxalta Österreich GmbH Tel.: +43 1 20100-0 |
España Baxalta Spain S.L. Tel: +34 91 790 42 22 | Polska Baxter Polska Sp. z o.o. Tel.: +48 22 4883 777 |
France Baxalta France S.A.S. Tél: +33 1 70 96 06 00 | Portugal Baxalta Portugal, Unipessoal, Lda. Tel: +351 21 122 03 00 |
Hrvatska Baxter d.o.o. Tel: +386 1 420 16 80 | România FARMACEUTICA REMEDIA SA Tel.: +40 21 321 16 40 |
Ireland Baxalta UK Limited Tel: +44 1 635 798 777 | Slovenija Baxter d.o.o. Tel.: +386 1 420 16 80 |
Ísland Lyfjaver ehf. Sími: +354 533 6100 | Slovenská republika Baxter Slovakia s.r.o. Tel: +421 2 3210 1150 |
Italia Baxalta Italy S.r.l. Tel: +39 06 45224 600 | Suomi/Finland Baxalta Finland Oy Puh/Tel: +358 201478200 |
Κύπρος Baxter (Hellas) Ε.Π.Ε. Τηλ.: +30 210 28 80 000 | Sverige Baxalta Sweden AB Tel: +46 8 50 53 26 00 |
Latvija SIA BAXTER Latvia Tel.: +371 67 784 784 | United Kingdom Baxalta UK Limited Tel: +44 1 635 798 777 |
Date of Last Revision of this Summary
Detailed information on this medicinal product is available on the European Medicines Agency web site: http://www.ema.europa.eu.
-----
This information is intended only for healthcare professionals:
Treatment Monitoring
During treatment, it is recommended to determine the appropriate levels of factor IX to calculate the dose to be administered and the frequency of repeated infusions. Individual patients may differ in their response to factor IX with different half-lives and recoveries. The dose based on body weight may require adjustment in patients with low weight or overweight. In the case of major surgical interventions, precise monitoring of replacement therapy through coagulation analysis (plasma factor IX activity) is essential.
To ensure that the desired plasma level of factor IX activity has been achieved, it is recommended to perform thorough monitoring using an appropriate factor IX activity assay and, if necessary, to apply the appropriate adjustments to the dose and frequency of repeated infusions. When using the in vitro coagulation assay in one stage based on the activated partial thromboplastin time (aPTT) to determine factor IX activity in patient blood samples, the results of factor IX activity may be significantly affected by the type of aPTT reagent and reference standard used in the assay. This is especially important when changing the laboratory and/or reagents used in the assay.
Dosage
The dose and duration of replacement therapy depend on the severity of the factor IX deficiency, the location and extent of the hemorrhage, as well as the patient's clinical condition, age, and pharmacokinetic parameters of factor IX, such as incremental recovery and half-life.
The number of units of factor IX administered is expressed in international units (IU), which are related to the current WHO standard for factor IX products. Factor IX activity in plasma is expressed as a percentage (relative to normal human plasma) or in international units (relative to an international standard for factor IX in plasma).
One international unit (IU) of factor IX activity is equivalent to the amount of factor IX present in one milliliter of normal human plasma.
On-demand Treatment
The calculation of the required dose of factor IX is based on the empirical finding that 1 international unit (IU) of factor IX per kilogram of body weight increases the factor IX activity of plasma by 0.9 IU/dl (range of 0.5 to 1.4 IU/dl) or 0.9% of normal activity in patients 12 years of age and older (for additional information, see section 5.2).
The required dosage is determined using the following formula:
Patients 12 years of age and older:
Units required | = | body weight (kg) | x | desired increase in factor IX (%) or (IU/dl) | x | reciprocal of observed recovery (dl/kg) |
For an incremental recovery of 0.9 IU/dl per IU/kg, the dose is calculated as follows:
Units required | = | body weight (kg) | x | desired increase in factor IX (%) or (IU/dl) | x | 1.1 dl/kg |
The amount to be administered and the frequency of administration should always be guided by the clinical efficacy in the specific case.
In the case of subsequent hemorrhagic episodes, factor IX activity should not be lower than the given plasma activity level (in % of normal or IU/dl) during the corresponding period. The following table can be used as a dosing guide for hemorrhagic episodes and surgery:
Severity of Hemorrhage / Type of Surgical Procedure | Required Factor IX Level (%) or(IU/dl) | Dose Frequency (hours) / Duration of Therapy (days) |
Hemorrhage Initial hemarthrosis or muscle or oral hemorrhage More extensive hemarthrosis, muscle hemorrhage, or hematoma Life-threatening hemorrhage. | 20 – 40 30 – 60 60 – 100 | Repeat every 24 hours. At least 1 day, until the hemorrhagic episode, as indicated by pain, is resolved or healing is achieved. Repeat infusion every 24 hours for 3 – 4 days or more, until pain and acute disability cease. Repeat infusion every 8 to 24 hours until the danger has passed. |
Surgery Minor surgery, including dental extraction | 30 – 60 | Every 24 hours, at least 1 day, until healing is achieved. |
Major Surgery | 80 – 100 (pre- and post-operative) | Repeat infusion every 8 to 24 hours until adequate wound healing is achieved, and then for at least another 7 days of therapy to maintain a factor IX activity of 30% to 60% (IU/dl). |
It is especially important to carefully monitor replacement therapy in cases of major surgery or potentially life-threatening hemorrhage.
Prophylaxis
For long-term prophylaxis against hemorrhages in patients with severe hemophilia B, the usual doses are 40 to 60 IU of factor IX per kilogram of body weight at intervals of 3 to 4 days for patients 12 years of age and older. In some cases, depending on pharmacokinetic parameters, age, hemorrhage phenotype, and patient physical activity, shorter dosing intervals or higher doses may be required.
Continuous Infusion
Do not administer RIXUBIS by continuous infusion.
Pediatric Population
On-demand treatment:
The calculation of the required dose of factor IX is based on the empirical finding that 1 international unit (IU) of factor IX per kilogram of body weight increases the factor IX activity of plasma by 0.7 IU/dl (range of 0.31 to 1.0 IU/dl) or 0.7% of normal activity in patients under 12 years of age (for additional information, see section 5.2).
The required dosage is determined using the following formula:
Patients under 12 years of age:
Units required | = | body weight (kg) | x | desired increase in factor IX (%) or (IU/dl) | x | reciprocal of observed recovery (dl/kg) |
For an incremental recovery of 0.7 IU/dl per IU/kg, the dose is calculated as follows:
Units required | = | body weight (kg) | x | desired increase in factor IX (%) or (IU/dl) | x | 1.4 dl/kg |
The same table for adults can be used as a dosing guide for hemorrhagic episodes and surgery (see above).
Prophylaxis:
The recommended dosing interval for pediatric patients under 12 years of age is 40 to 80 IU/kg at intervals of 3 to 4 days. In some cases, depending on pharmacokinetic parameters, age, hemorrhage phenotype, and patient physical activity, shorter dosing intervals or higher doses may be required.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Rixubis 250 IU/vial powder and solvent for injectable solutionDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 2,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for Rixubis 250 IU/vial powder and solvent for injectable solution
Discuss questions about Rixubis 250 IU/vial powder and solvent for injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















