
RIVASTIGMINE CINFA 13.3 mg/24h TRANSDERMAL PATCHES

How to use RIVASTIGMINE CINFA 13.3 mg/24h TRANSDERMAL PATCHES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Rivastigmine Cinfa 13.3 mg/24 h Transdermal Patches EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Rivastigmine Cinfa and what is it used for.
- What you need to know before you start using Rivastigmine Cinfa.
- How to use Rivastigmine Cinfa.
- Possible side effects.
- Storing Rivastigmine Cinfa.
- Contents of the pack and further information.
1. What is Rivastigmine Cinfa and what is it used for
The active substance of Rivastigmine Cinfa is rivastigmine.
Rivastigmine belongs to a group of medicines called cholinesterase inhibitors. In patients with Alzheimer's dementia, certain nerve cells die in the brain, leading to low levels of the neurotransmitter acetylcholine (a substance that allows nerve cells to communicate with each other). Rivastigmine works by blocking the enzymes that break down acetylcholine: acetylcholinesterase and butyrylcholinesterase. By blocking these enzymes, Rivastigmine Cinfa allows an increase in acetylcholine in the brain, helping to reduce the symptoms of Alzheimer's disease.
Rivastigmine Cinfa is used for the treatment of adult patients with mild to moderately severe Alzheimer's dementia, a progressive brain disorder that gradually affects memory, intellectual ability, and behavior.
2. What you need to know before you start using Rivastigmine Cinfa
Do not use Rivastigmine Cinfa
- if you are allergic to rivastigmine or any of the other ingredients of this medicine (listed in section 6).
- if you have ever had an allergic reaction to a similar medicine (carbamate derivatives).
- if you have a skin reaction that spreads beyond the size of the patch, if there is a more intense local reaction (such as blisters, increasing skin inflammation, swelling), and if there is no improvement within 48 hours after removing the transdermal patch.
If you are in any of these situations, inform your doctor and do not use rivastigmine transdermal patches.
Warnings and precautions
Consult your doctor or pharmacist before starting to use rivastigmine:
- If you have or have ever had any heart problems, such as an irregular or slow heartbeat, QTc prolongation, family history of QTc prolongation, torsades de pointes, or if you have low levels of potassium or magnesium in your blood.
- If you have or have ever had an active stomach ulcer.
- If you have or have ever had difficulty urinating.
- If you have or have ever had seizures.
- If you have or have ever had asthma or severe respiratory disease.
- If you suffer from tremors.
- If you have low body weight.
- If you have gastrointestinal reactions such as a feeling of nausea, vomiting, and diarrhea. You may become dehydrated (lose a large amount of fluid) if vomiting or diarrhea is prolonged.
- If you have liver problems (liver failure).
If you are in any of these situations, your doctor may consider it necessary to monitor you more closely while you are being treated.
If you have not used the patches for more than three days, do not put on another one without consulting your doctor first.
Children and adolescents
This medicine must not be used in the pediatric population for the treatment of Alzheimer's disease.
Other medicines and Rivastigmine Cinfa
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Rivastigmine may interfere with anticholinergic medicines, some of which are medicines used to relieve stomach cramps or spasms (e.g., dicyclomine), for the treatment of Parkinson's disease (e.g., amantadine), or to prevent motion sickness (e.g., diphenhydramine, scopolamine, or meclizine).
This medicine must not be given at the same time as metoclopramide (a medicine used to relieve or prevent nausea and vomiting). Taking the two medicines together may cause problems such as stiffness in the limbs and hand tremors.
If you are going to have surgery while using rivastigmine transdermal patches, inform your doctor that you are using it, as it may potentiate the effects of some muscle relaxants used in anesthesia.
Caution should be exercised when using this medicine with beta-blockers (medicines such as atenolol used to treat high blood pressure, angina, and other heart conditions). Taking the two medicines together may cause complications such as a decrease in heart rate (bradycardia) that can lead to fainting or loss of consciousness.
Caution should be exercised when using rivastigmine with other medicines that may affect heart rhythm or the heart's electrical system (QT prolongation).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
If you are pregnant, it is necessary to evaluate the benefits of using rivastigmine against the possible adverse effects for the fetus. Rivastigmine should not be used during pregnancy unless clearly necessary.
You should not breastfeed while being treated with this medicine.
Driving and using machines
Your illness may affect your ability to drive or use machines, and you should not carry out these activities unless your doctor tells you it is safe to do so. Rivastigmine may cause dizziness and somnolence, mainly at the start of treatment or when increasing the dose. If you experience these effects, do not drive or use machines.
3. How to use Rivastigmine Cinfa
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
IMPORTANT:
- Remove the previous patch before putting on a NEW patch.
- ONLY ONE patch per day.
- Do NOT cut the patch into pieces.
- Press the patch firmly against the skin with the palm of your hand for at least 30 seconds.
How to start treatment
Your doctor will tell you which dose of rivastigmine transdermal patches is most suitable for you.
- Normally, treatment is started with rivastigmine 4.6 mg/24 h.
- The recommended daily dose is rivastigmine 9.5 mg/24 h. If this dose is well tolerated, the doctor treating you may consider increasing the dose to 13.3 mg/24 h.
- Only wear one rivastigmine patch at a time and replace the patch with a new one after 24 hours.
During treatment, your doctor may adjust the dose depending on your individual needs.
If you have not used the patches for more than three days, do not put on another one without consulting your doctor first. Treatment with the transdermal patch can be restarted at the same dose if treatment is not interrupted for more than three days. Otherwise, your doctor will have you restart your treatment with rivastigmine 4.6 mg/24 h.
Rivastigmine can be used with food, drinks, and alcohol.
Where to apply your Rivastigmine Cinfa transdermal patch
- Before applying a patch, make sure the skin is clean, dry, and hairless, without powders, oils, moisturizers, or lotions that could prevent the patch from sticking well to the skin, without cuts, redness, or irritation.
- Remove carefully any patch you are already wearing before putting on a new one.Wearing multiple patches on your body could expose you to an excessive amount of this medicine, which could be potentially dangerous.
- Apply ONLY ONEpatch per day to ONLY ONEof the possible areas as shown in the following diagrams:
- upper left orupper right arm
- upper left orupper right chest (avoiding the breasts in women)
- upper left orupper right back
- lower left or lower right back
Every 24 hours, remove the previous patch before putting on a NEW patch in ONLY ONE of the following possible areas
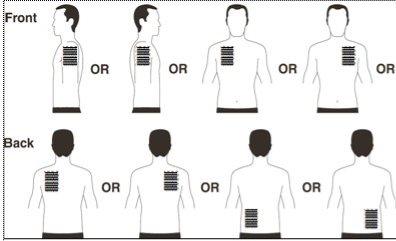
Each time you change the patch, you must remove the previous day's patch before applying a new patch to a different area of skin (e.g., one day on the right side of the body and the next day on the left side; or one day on the upper part of the body and the next day on the lower part). Wait at least 14 days before applying a new patch to exactly the same area of skin.
How to apply your Rivastigmine Cinfa transdermal patches
The rivastigmine patches are thin, opaque plastic and stick to the skin. Each patch is in a pouch that protects it until you are ready to apply it. Do not open the pouch or remove the patch until you are ready to apply it.
Remove carefully any existing patch before applying a new one.
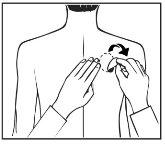
Patients who are starting treatment for the first time and patients who are restarting treatment with rivastigmine after an interruption of treatment must start with the second figure.
- Each patch is in an individual protective pouch. Only open the pouch when you are ready to apply the patch. Cut the pouch along the dotted line with scissors and remove the patch from the pouch.
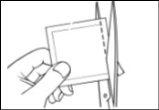
- A divided protective liner covers the adhesive side of the patch. Remove the first liner half without touching the adhesive side of the patch with your fingers.
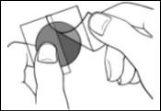
- Place the adhesive side of the patch on the upper or lower back or on the upper arm or chest (avoiding the breasts in women)and then remove the second liner half.
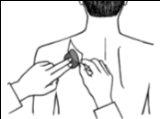
- Press the patch firmly against the skin with the palm of your hand for at least 30 seconds and make sure the edges are well stuck.
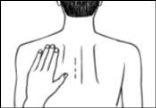
- If it helps, you can write on the patch, for example, the day of the week, with a fine-tipped pen.
You must wear the patch continuously until it is time to change it for a new one. When you apply a new patch, you can try different areas to find the ones that are most comfortable for you and where your clothes do not rub against the patch.
How to remove your Rivastigmine Cinfa transdermal patch
Gently pull on one of the edges of the patch to slowly peel it off the skin. If adhesive residue remains on the skin, soak the area with warm water and mild soap or use baby oil to remove it. Do not use alcohol or other solvents (nail polish removers or other solvents).
After removing the patch, wash your hands with soap and water. If you get the patch in your eyes or if your eyes become red after handling the patch, rinse immediately with plenty of water and seek medical advice if the symptoms do not resolve.
Can you wear your Rivastigmine Cinfa transdermal patch when bathing, swimming, or exposing yourself to the sun?
- Bathing, swimming, or showering should not affect the patch. Make sure it does not peel off partially while you are doing these activities.
- Do not expose the patch to an external heat source (e.g., excessive sunlight, sauna, sunbed) for long periods.
What to do if a patch falls off
If a patch falls off, apply a new one for the rest of that day and change it the next day at the usual time.
When and for how long should you wear your Rivastigmine Cinfa transdermal patches
- To benefit from your treatment, you must apply a new patch every day, preferably at the same time.
- Only wear one Rivastigmine Cinfa patch at a time and replace the patch with a new one after 24 hours.
If you use more Rivastigmine Cinfa than you should
If you accidentally apply more than one patch, remove all patches from the skin and inform your doctor. You may need medical attention. Some people who have accidentally taken too high doses of rivastigmine orally have experienced nausea, vomiting, diarrhea, high blood pressure, and hallucinations. There may also be a slowing of the heart rate and fainting.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Rivastigmine Cinfa
If you realize you have forgotten to apply a patch, apply it immediately. The next day, apply the next patch at the usual time. Do not apply two patches to make up for the one you forgot.
If you stop using Rivastigmine Cinfa
Tell your doctor or pharmacist if you stop using the patches.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
You may have adverse effects more frequently when starting your treatment or when your dose is increased. Generally, adverse effects will slowly disappear as your body gets used to the medicine.
If you notice any of the following adverse effects that may be serious, remove the patch and inform your doctor immediately.
Frequent(may affect up to 1 in 10 people)
- Lack of appetite
- Dizzy sensation
- Feeling of agitation or numbness
- Urinary incontinence (inability to stop urine properly).
Uncommon(may affect up to 1 in 100 people)
- Heart rhythm problems such as slow heart rate
- Seeing things that do not really exist (hallucinations)
- Stomach ulcer
- Dehydration (loss of a large amount of fluid)
- Hyperactivity (high level of activity, restlessness)
- Aggressiveness
Rare(may affect up to 1 in 1,000 people)
- Falls
Very Rare(may affect up to 1 in 10,000 people)
- Stiffness of the arms and legs
- Tremor in the hands
Frequency Not Known(cannot be estimated from the available data)
- Allergic reaction where the patch was applied, such as blisters or skin inflammation
- Worsening of Parkinson's disease signs – such as tremor, stiffness, and difficulty moving
- Pancreatitis – signs include pain in the upper part of the stomach, often accompanied by a feeling of dizziness (nausea) or vomiting
- Fast or irregular heart rate
- High blood pressure
- Seizures (convulsions)
- Liver disorders (yellowing of the skin, yellowing of the whites of the eyes, abnormal darkening of urine or unexplained nausea, vomiting, fatigue, and loss of appetite)
- Changes in analyzes that show liver function
- Feeling of restlessness
- Nightmares
If you notice any of the adverse effects listed above, remove the patch and inform your doctor immediately.
Other Adverse Effects Experienced with Rivastigmine Capsules or Oral Solution and That May Occur with Patches:
Frequent(may affect up to 1 in 10 people)
- Excessive saliva
- Lack of appetite
- Feeling of agitation
- Feeling of general discomfort
- Tremor or feeling of confusion
- Increased sweating
Uncommon(may affect up to 1 in 100 people)
- Irregular heart rate (e.g., fast heart rate)
- Difficulty sleeping
- Accidental falls
Rare(may affect up to 1 in 1,000 people)
- Seizures (convulsions)
- Ulcer in the intestine
- Chest pain – probably caused by spasm in the heart
Very Rare(may affect up to 1 in 10,000 people)
- High blood pressure
- Pancreatitis – signs include severe pain in the upper part of the stomach, often with a feeling of dizziness (nausea) or vomiting
- Gastrointestinal bleeding – manifested as blood in the stool or when vomiting
- Seeing things that do not exist (hallucinations)
- Some people who have been intensely dizzy (vomiting) have had a tear in part of the digestive tube that connects their mouth to their stomach (esophagus)
Frequency Not Known(cannot be estimated from the available data)
- Pisa syndrome (a condition that involves involuntary muscle contraction and abnormal tilting of the body and head to one side)
Patients with Dementia or Parkinson's Disease
These patients experience some adverse effects more frequently and also have some additional adverse effects:
Frequency Not Known(cannot be estimated from the available data)
- Pisa syndrome (a condition that involves involuntary muscle contraction and abnormal tilting of the body and head to one side)
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Rivastigmine Cinfa
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and on the envelope after CAD. The expiration date is the last day of the month indicated.
Do not use any patch if you observe that it is damaged or shows signs of tampering.
Store in the original packaging to protect it from light.
Store the transdermal patch in the envelope until use.
After removing a patch, fold it in half with the adhesive side facing inward and press. After introducing it into the original envelope, when disposing of the patch, make sure it is out of sight and reach of children. After removing the patch, do not touch your eyes, and wash your hands well with soap and water.
Medicines should not be thrown away through drains or into the trash. Deposit the containers and medicines you no longer need in the SIGRE Point of the pharmacy. Ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Rivastigmine Cinfa
- The active principle is rivastigmine. Each patch contains 27 mg of rivastigmine in 15 cm2 and releases 13.3 mg of rivastigmine in 24 hours.
- The other components are: polyester film, polyester film coated with silica, fluorinated polyester film, acrylic adhesive (Duro-Tak 87-235A), acrylate copolymer (Plastoid B), ethyl acetate, silicone adhesive (Bio-PSA 7-4302), and ink.
Appearance of the Product and Package Contents
Rivastigmine Cinfa 13.3 mg/24 h transdermal patches are presented in the form of a transdermal patch composed of a rounded adhesive and a rectangular support film. Each envelope contains a transdermal patch.
Rivastigmine Cinfa 13.3 mg/24 h transdermal patches are available in packages containing 60 envelopes.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) - Spain
Manufacturer
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta
31620 Huarte (Navarra) - Spain
or
Eurofins PHAST GmbH
Kardinal-Wendel-Strasse 16,
66424 Homburg, Germany
or
ACC GmbH Analytical Clinical Concepts
Schöntalweg 9,
63849 Leidersbach, Germany
Date of the Last Revision of this Prospectus:December 2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
You can access detailed and updated information about this medicine by scanning the QR code included in the prospectus and packaging with your mobile phone (smartphone). You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/80657/P_80657.html
QR code to: https://cima.aemps.es/cima/dochtml/p/80657/P_80657.html
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RIVASTIGMINE CINFA 13.3 mg/24h TRANSDERMAL PATCHESDosage form: TRANSDERMAL PATCH, 13.3 mg/24 hActive substance: rivastigmineManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: TRANSDERMAL PATCH, 4.6 mg/24 hActive substance: rivastigmineManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: TRANSDERMAL PATCH, 9.5 mg/24 hActive substance: rivastigmineManufacturer: Esteve Pharmaceuticals S.A.Prescription required
Online doctors for RIVASTIGMINE CINFA 13.3 mg/24h TRANSDERMAL PATCHES
Discuss questions about RIVASTIGMINE CINFA 13.3 mg/24h TRANSDERMAL PATCHES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













