
RISPERIDONE TEVA 1 mg/ml ORAL SOLUTION

How to use RISPERIDONE TEVA 1 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Risperidone Teva1mg/ml Oral Solution EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Risperidone Teva and what is it used for
- What you need to know before you take Risperidone Teva
- How to take Risperidone Teva
- Possible side effects
- Storing Risperidone Teva
- Contents of the pack and other information
1. What is Risperidone Teva and what is it used for
Risperidone Teva belongs to a group of medicines called "antipsychotics".
Risperidone is used to treat the following:
- Schizophrenia, where you may see, hear, or feel things that are not there, believe things that are not true, or feel unusually suspicious or confused.
- Mania, where you may feel very excited, elated, agitated, enthusiastic, or hyperactive. Mania occurs in a condition called "bipolar disorder".
- Short-term treatment (up to 6 weeks) of persistent aggression in people with Alzheimer's dementia, who may harm themselves or others. Other non-pharmacological treatments should have been tried before.
- Short-term treatment (up to 6 weeks) of persistent aggression in intellectually disabled children (at least 5 years of age) and adolescents with conduct disorders.
Risperidone may help to decrease the symptoms of your illness and prevent them from returning.
2. What you need to know before you take Risperidone Teva
Do not take Risperidone Teva:
- if you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6).
If you are not sure, consult your doctor or pharmacist before taking Risperidone Teva.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Risperidone if:
- You have any heart problems. Examples include irregular heartbeats, or if you are prone to having low blood pressure or if you are taking medicines for blood pressure. Risperidone may lower your blood pressure. You may need to have your dose adjusted.
- You know of any factor that may make you prone to having a stroke, such as high blood pressure, cardiovascular diseases, or problems with the blood vessels in the brain.
- You have ever had involuntary movements of the tongue, mouth, and face.
- You have ever had symptoms that include fever, stiff muscles, sweating, or a decreased level of consciousness (also known as Neuroleptic Malignant Syndrome).
- You have Parkinson's disease or dementia.
- You have had low white blood cell counts in the past (which may or may not have been caused by other medicines).
- You are diabetic.
- You have epilepsy.
- You are male and have ever had a prolonged or painful erection. If you experience this while taking Risperidone, contact your doctor immediately.
- You have problems controlling your body temperature or feel excessive heat.
- You have kidney problems.
- You have liver problems.
- You have an abnormally high level of a hormone called prolactin in your blood or if you have a tumor that may be dependent on prolactin.
- If you or a family member have a history of blood clotting problems, as antipsychotics have been associated with the formation of blood clots.
If you are unsure about any of the above, consult your doctor or pharmacist before taking Risperidone Teva.
Because it has been very rarely observed in patients treated with Risperidone that there is a dangerously low number of a type of white blood cell necessary to fight infections in the blood, your doctor may check your white blood cell count.
Risperidone may cause you to gain weight. A significant weight gain can affect your health unfavorably. Your doctor will regularly monitor your weight.
Because diabetes mellitus or worsening of pre-existing diabetes mellitus has been seen in patients taking Risperidone, your doctor should check for signs of high blood sugar. In patients with pre-existing diabetes mellitus, blood sugar should be regularly monitored.
Risperidone often increases the levels of a hormone called prolactin. This can cause side effects such as menstrual disorders or fertility problems in women or breast swelling in men (see "Possible side effects"). If these side effects occur, it is recommended to evaluate the prolactin levels in the blood.
During eye surgery for cataracts, the pupil (the black circle in the middle of the eye) may not increase in size as needed. Additionally, the iris (the colored part of the eye) may become flaccid during surgery, which can cause eye damage. If you are planning to have eye surgery, make sure to inform your ophthalmologist that you are using this medicine.
Elderly patients with dementia
In elderly patients with dementia, there is an increased risk of having a stroke. You should not take Risperidone if you have dementia caused by a stroke.
During treatment with Risperidone, you should see your doctor frequently.
If you or your caregiver notice a sudden change in your mental state or the sudden appearance of weakness or numbness in your face, arms, or legs, especially on one side, or confused speech, even if only for a short time, seek medical treatment immediately. It may be a sign of a stroke.
Children and adolescents
Other causes of aggressive behavior should be ruled out before starting treatment for conduct disorders.
If during treatment with Risperidone you experience fatigue, changing the administration times may improve your attention difficulties.
Before starting treatment, your weight or your child's weight may be measured and may be regularly monitored during treatment.
A small, inconclusive study has reported an increase in height in children who took Risperidone, but it is unknown whether this is an effect of the drug or due to another reason.
Other medicines and Risperidone Teva
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
It is especially important to talk to your doctor or pharmacist if you are taking any of the following medicines:
- Medicines that act on your brain, such as those used to calm you down (benzodiazepines) or some pain medicines (opioids), medicines for allergies (some antihistamines), as Risperidone may increase their sedative effect.
- Medicines that can change the electrical activity of your heart, such as those used for malaria, heart rhythm problems, allergies (antihistamines), some antidepressants, or other medicines for mental problems.
- Medicines that cause a slow heartbeat.
- Medicines that cause low potassium levels in the blood (e.g., some diuretics).
- Medicines for high blood pressure. Risperidone may lower your blood pressure.
- Medicines for Parkinson's disease (such as levodopa).
- Medicines that increase the activity of the central nervous system (psychostimulants, such as methylphenidate).
- Diuretics, which are used for heart problems or to treat swelling of some parts of the body due to fluid retention (such as furosemide or chlorthiazide). Risperidone, taken alone or with furosemide, may increase the risk of stroke or death in elderly people with dementia.
The following medicines may decrease the effect of Risperidone:
- Rifampicin (a medicine for treating some infections).
- Carbamazepine, phenytoin (medicines for epilepsy).
- Phenobarbital.
If you start or stop taking these medicines, you may need a different dose of Risperidone.
The following medicines may increase the effect of Risperidone:
- Quinidine (used for certain types of heart diseases).
- Antidepressants such as paroxetine, fluoxetine, and tricyclic antidepressants.
- Medicines known as beta-blockers (used to treat high blood pressure).
- Phenothiazines (such as medicines used to treat psychosis or as sedatives).
- Cimetidine, ranitidine (stomach acid blockers).
- Itraconazole and ketoconazole (medicines used to treat fungal infections).
- Some medicines used to treat HIV/AIDS, such as ritonavir.
- Verapamil, a medicine used to treat high blood pressure and/or irregular heartbeat.
- Sertraline and fluvoxamine, medicines used to treat depression and other psychiatric disorders.
If you start or stop taking these medicines, you may need a different dose of Risperidone.
If you are unsure about any of the above, consult your doctor or pharmacist before taking Risperidone Teva.
Taking Risperidone Teva with food, drinks, and alcohol
You can take this medicine with or without food. You should avoid consuming alcohol while taking Risperidone.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Your doctor will decide whether you can take it.
- The following symptoms may occur in newborn babies of mothers who have been treated with Risperidone in the last trimester (last three months of pregnancy): shaking, muscle stiffness and/or weakness, drowsiness, agitation, breathing problems, and feeding difficulties. If your newborn baby develops any of these symptoms, you should contact your doctor.
- Risperidone may increase the levels of a hormone called "prolactin" that can affect fertility (see Possible side effects).
Consult your doctor or pharmacist before taking any medicine.
Driving and using machines
Dizziness, tiredness, and vision problems have been observed during treatment with Risperidone. Do not drive or operate tools or machines without consulting your doctor first.
Risperidone Teva1 mg/ml Oral Solution contains sorbitol (E 420)
This medicine contains 150 mg of sorbitol in each ml of oral solution. Sorbitol is a source of fructose. If your doctor has told you (or your child) that you have an intolerance to some sugars, or you have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disorder in which the patient cannot break down fructose, consult your doctor before taking this medicine.
Risperidone Teva1 mg/ml Oral Solution contains benzoic acid (E 210)
This medicine contains 2 mg of benzoic acid in each ml of oral solution.
3. How to take Risperidone Teva
Follow exactly the administration instructions of this medication as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is as follows:
For the treatment of schizophrenia
Adults
- The starting dose is 2 mg per day, which can be increased to 4 mg per day on the second day.
- Your doctor may adjust your dose depending on how you respond to treatment.
- Most people feel better with daily doses of 4 to 6 mg.
- This total daily dose can be divided into one or two doses per day. Your doctor will indicate what is best for you.
Elderly patients
- The starting dose will normally be 0.5 mg, twice a day.
- Later, your doctor will gradually increase your dose to 1 mg - 2 mg, twice a day.
- Your doctor will indicate what is best for you.
Children and adolescents
- Children and adolescents under 18 years of age should not receive treatment with risperidone for schizophrenia.
For the treatment of mania
Adults
- The starting dose will normally be 2 mg, once a day.
- Your doctor may adjust your dose gradually later, depending on your response to treatment.
- Most people feel better with daily doses of 1 to 6 mg.
Elderly patients
- The starting dose will be 0.5 mg, twice a day, normally.
- Your doctor may adjust your dose gradually later to 1 mg - 2 mg, twice a day, depending on your response to treatment.
Children and adolescents
- Children and adolescents under 18 years of age should not receive treatment with risperidone for bipolar mania.
For the treatment of long-term aggression in people with Alzheimer's type dementia
Adults (including elderly patients)
- The starting dose will be 0.25 mg (0.25 ml of risperidone oral solution 1 mg/ml), twice a day, normally.
- Your doctor may change your dose gradually later, depending on your response to treatment.
- Most people feel better with doses of 0.5 mg, twice a day. Some patients may need 1 mg, twice a day.
- The duration of treatment in patients with Alzheimer's type dementia should not exceed 6 weeks.
For the treatment of behavioral disorders in children and adolescents
The dose will depend on your child's weight:
If they weigh less than50 kg
- The starting dose will normally be 0.25 mg (0.25 ml of risperidone oral solution 1 mg/ml), once a day.
- The dose can be increased, one day yes and one day no, in increments of 0.25 mg per day.
- The normal maintenance dose is 0.25 mg to 0.75 mg (0.25 ml of risperidone oral solution 1 mg/ml), once a day.
If they weigh50 kgor more
- The starting dose will normally be 0.5 mg, once a day.
- The dose can be increased, one day yes and one day no, in increments of 0.5 mg per day.
- The normal maintenance dose is 0.5 mg to 1.5 mg, once a day.
The duration of treatment in patients with behavioral disorders should not exceed 6 weeks.
Children under 5 years of age should not receive treatment with risperidone for behavioral disorders.
Patients with liver or kidney problems
Regardless of the disease being treated, all starting doses and subsequent doses of risperidone should be reduced by half. Dose increases should be made more slowly in these patients.
Risperidone should be used with caution in this group of patients.
Method of administration
Oral route.
How to take Risperidone Teva
Always take risperidone exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Your doctor will indicate how much medication you should take and for how long. This will depend on your disease and varies from person to person. The amount of medication you should take is explained above in the "How much to take" section.
Risperidone Teva oral solution
The solution comes with a syringe (pipette). Use only the syringe (pipette) provided with this medication to measure the prescribed dose. Measure the exact dose of the medication you need. Pay attention when measuring a small dose, for example, for 0.25 mg, you need to measure 0.25 ml (a quarter of a milliliter); for 0.5 mg, you need to measure 0.5 ml (half a milliliter).
Follow these steps:
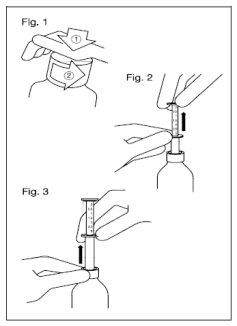
- Remove the child-resistant cap. Push the plastic cap down, turning it counterclockwise (Figure 1).
- Insert the syringe into the bottle.
- Holding the lower ring, pull the upper ring up to the mark that corresponds to the number of milliliters or mg you should administer (Figure 2).
- Holding the lower ring, remove the entire syringe from the bottle (Figure 3).
- Empty the syringe into a non-alcoholic drink that is not tea. Slide the upper ring down.
- Close the bottle.
- Wash the syringe with water and let it air dry.
If you take more Risperidone Teva than you should
- Go to the doctor immediately. Take the medication package with you. You can also call the Toxicology Information Service, phone 91 562 04 20.
- In case of overdose, you may feel drowsy or tired, have abnormal body movements, problems standing and walking, dizziness due to low blood pressure, or have abnormal heartbeats or convulsions.
If you forget to take Risperidone Teva
- If you forget to take a dose, take it as soon as you remember. But if it's almost time for the next dose, skip the missed dose and continue as normal. If you forget two or more doses, contact your doctor.
- Do not take a double dose (two doses at the same time) to make up for missed doses.
If you stop treatment with Risperidone Teva
You should not stop taking the treatment unless your doctor tells you to. Symptoms may reappear. If your doctor decides to stop the treatment, your dose can be gradually reduced over a few days.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medication can cause adverse effects, although not all people suffer from them.
Report immediately to your doctor if you experience any of the following rare adverse effects (may affect up to 1 in 100 patients):
- You have dementia and experience a sudden change in your mental state or sudden weakness or numbness of the face, arms, or legs, especially on one side, or have difficulty speaking even for a short period. These may be signs of a stroke.
- You experience tardive dyskinesia (involuntary spasms or movements that cannot be controlled in the face, tongue, or other parts of the body). Report to your doctor immediately if you experience involuntary rhythmic movements of the tongue, mouth, and face. It may be necessary to withdraw risperidone.
Report immediately to your doctor if you experience any of the following rare adverse effects (may affect up to 1 in 1,000 patients):
- You experience blood clots in the veins, especially in the legs (symptoms include swelling, pain, and redness of the leg), which can circulate through the blood vessels to the lungs, causing chest pain and difficulty breathing. If you notice any of these symptoms, seek medical advice immediately.
- You experience fever, muscle stiffness, sweating, or a decrease in the level of consciousness (a condition known as "Neuroleptic Malignant Syndrome"). You may need immediate medical treatment.
- You are a man and experience a prolonged or painful erection. This is known as priapism. You may need immediate medical treatment.
- You experience a severe allergic reaction characterized by fever, swelling of the mouth, face, lips, or tongue, difficulty breathing, itching, skin rash, or low blood pressure.
The following side effects may also occur:
Very common adverse effects (may affect more than 1 in 10 people)
- Parkinsonism: This disease may include slow or altered movement, feeling of stiffness or tension in the muscles (making jerky movements), and sometimes a feeling of "freezing" of movement that then restarts. Other signs of parkinsonism include walking slowly, dragging feet, tremors at rest, increased saliva and/or drooling, and loss of facial expressiveness.
- Difficulty staying or falling asleep.
- Feeling drowsy or less attentive.
- Headache.
Common adverse effects (may affect up to 1 in 10 people):
- Fatigue, tiredness, restlessness, irritability, anxiety, dizziness, feeling of exhaustion, sleep disorders, depression.
- Vomiting, diarrhea, constipation, nausea, abdominal pain or discomfort, sore throat, dry mouth, toothache, indigestion.
- Dyskinesia: This disease involves involuntary muscle movements and may include repetitive, spasmodic, or twisting movements, or spasms.
- Dystonia: This is a disease that involves slow or continuous involuntary contraction of the muscles. Although any part of the body can be affected (and may cause abnormal postures), dystonia often affects the muscles of the face, including abnormal movements of the eyes, mouth, tongue, or jaw.
- Tremors (shakiness).
- Weight gain, increased appetite, decreased appetite.
- Sinusitis, lung infection (pneumonia), symptoms of a common cold, feeling like you have the flu, chest infection (bronchitis), nasal congestion, nosebleeds, cough, ear infection.
- Urinary tract infection, urinary incontinence (loss of control).
- Muscle spasms, bone or muscle pain, joint pain, back pain, swelling of the body, arms, or legs, fever, chest pain, weakness, fatigue (tiredness), pain.
- Rash, skin redness.
- Fast heartbeat, high blood pressure, shortness of breath.
- Increased levels of the hormone prolactin in the blood (which may or may not cause symptoms). Symptoms of high prolactin levels occur rarely and may include in men, breast swelling, difficulty having or maintaining erections, or other sexual dysfunctions. In women, they may include breast discomfort, milk secretion from the breasts, loss of menstrual periods, or other problems with the menstrual cycle or fertility issues.
- Blurred vision, eye infection, or "red eye."
- Falls.
Uncommon adverse effects (may affect up to 1 in 100 people):
- Fecal incontinence, feeling of thirst, very hard stools, excess gas or flatulence.
- Respiratory tract infection, bladder infection, eye redness, viral infection, tonsillitis, skin infection, eye infection, stomach or intestine infection, fungal infection of the nails.
- Atrial fibrillation (abnormal heart rhythm), interruption of conduction between the upper and lower parts of the heart, anomaly in the electrical conduction of the heart, low blood pressure when standing up, as a result, some people taking risperidone may feel weakness, dizziness, or loss of consciousness when standing up or sitting down suddenly, flushing, low blood pressure, feeling of dizziness when changing position, prolonged QT interval in the heart, anomaly of heart electrical activity (electrocardiogram or ECG), abnormal heart rhythm, feeling of fluttering or pounding in the chest (palpitations), slow heartbeat.
- Painful urination, frequent urination, inability to urinate.
- Confusion, altered attention, decreased level of consciousness, nervousness, euphoria (mania), nightmares.
- Seizures (epileptic fits).
- Diabetes or worsening of diabetes, high blood sugar, excessive water intake.
- High cholesterol in the blood.
- Increased liver enzymes in the blood, increased GGT (a liver enzyme called gamma-glutamyltransferase) in the blood, increased liver enzymes in the blood.
- Decreased number of white blood cells, decreased hemoglobin or red blood cell count (anemia), increased number of eosinophils (special white blood cells), increased creatine phosphokinase (CPK) in the blood, an enzyme that is sometimes released with muscle breakdown, decreased platelet count (blood cells that help stop bleeding).
- Muscle weakness, ear pain, neck pain, joint swelling, abnormal posture, joint stiffness, chest discomfort.
- Skin injury, skin disorder, skin dryness, intense itching, acne, hair loss, skin inflammation caused by mites, skin discoloration, skin thickening, scaly and itchy skin or scalp, localized infection in a single area of the skin or body part, reduced sensitivity of the skin to pain or touch.
- Absence of menstruation, sexual dysfunction, erectile dysfunction, ejaculation disorder, milk secretion from the breasts, breast enlargement in males, breast pain, breast discomfort, decreased sexual desire, loss of menstrual periods or other problems with the menstrual cycle (in women), vaginal discharge.
- Fainting, change in gait, weight loss, loss of appetite with malnutrition and weight loss, feeling of discomfort, discomfort, feeling "unwell," balance disorder, feeling of spinning or swaying (vertigo), allergic reaction, eczema, hives (or "urticaria"), speech problems, loss or alteration of taste, chills, increased body temperature, coordination anomaly.
- A restlessness that causes movement of body parts, feeling of tingling, pins and needles, or numbness of the skin.
- Increased sensitivity of the eyes to light, dry eyes, increased tearing.
- Breathing difficulties, wheezing, pulmonary congestion, crackling sounds in the lungs, respiratory tract disorders, voice disorders, difficulty swallowing, aspiration pneumonia.
- Loss of response to stimuli, loss of consciousness, swelling of the face, mouth, lips, and eyes, ringing in the ears.
- Pain due to the procedure.
Rare adverse effects (may affect up to 1 in 1,000 people):
- Inability to reach orgasm, delayed menstruation, breast enlargement, milk secretion from the breasts.
- Dandruff.
- Sleepwalking.
- Sleep-related eating disorder.
- Lack of movement or response while awake (catatonia).
- Head shaking.
- Irregular heartbeat.
- Glaucoma (increased eye pressure), eye rotation, crust formation on the eyelid margin, eye movement problems.
- Lack of emotions.
- Decreased body temperature, coldness in arms and legs.
- Rapid and shallow breathing, respiratory problems during sleep (sleep apnea), intestinal obstruction.
- Problems with the blood vessels in the brain.
- Inadequate secretion of a hormone that controls urine volume.
- Sugar in the urine, low blood sugar, high triglycerides in the blood (a type of fat), high insulin levels in the blood (a hormone that controls blood sugar levels).
- Muscle fiber breakdown and muscle pain (rhabdomyolysis), movement disorder.
- Diabetic coma.
- Yellowing of the skin and eyes (jaundice).
- Pancreatitis.
- Infection.
- Eye problems during cataract surgery. During cataract surgery, a condition called intraoperative floppy iris syndrome (IFIS) may occur if you are taking or have taken risperidone. If you need to undergo cataract surgery, make sure to inform your ophthalmologist if you are taking or have taken this medication.
- Tongue swelling, cracked lips, skin rash related to the medication, dangerously low number of a type of white blood cell necessary to fight blood infections, excessively high water intake.
- Drug withdrawal syndrome.
Very rare adverse effects (may affect up to 1 in 10,000 people):
- Complications of uncontrolled diabetes, with risk to life.
- Severe allergic reaction with swelling, which can affect the throat, causing difficulty breathing.
- Intestinal obstruction (ileus).
Frequency not known: cannot be estimated from the available data
- Severe rash with blisters and peeling of the skin that can start around the mouth, nose, eyes, genitals, and spread to other areas of the body (Stevens-Johnson syndrome or toxic epidermal necrolysis).
The following adverse effect has been reported with the use of another medication called paliperidone, which is very similar to risperidone, and is also expected to occur with Risperidone: Fast heartbeat when standing up.
Risperidone Long-Acting Injectable
The following adverse effects have been reported with the use of Risperidone Long-Acting Injectable. If you experience any of the following effects, talk to your doctor, even if you are not receiving treatment with long-acting injections of Risperidone:
- Intestinal infection.
- Abscess under the skin, tingling, pins and needles, or numbness of the skin, skin inflammation.
- Decreased number of white blood cells, cells that help protect against bacterial infections.
- Depression.
- Seizures.
- Eyelid spasms.
- Feeling of spinning or swaying.
- Slow heartbeat, high blood pressure.
- Toothache, tongue spasm.
- Buttock pain.
- Weight loss.
Other adverse effects in children and adolescents
In general, it is expected that the adverse effects in children will be similar to those that occurred in adults. The following adverse effects were reported more frequently in children and adolescents (5 to 17 years) than in adults: feeling drowsy or less attentive, fatigue (tiredness), headache, increased appetite, vomiting, common cold symptoms, nasal congestion, abdominal pain, dizziness, cough, fever, tremors (shakiness), diarrhea, and urinary incontinence (loss of control).
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency (AEMPS) website: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Risperidone Teva
No special storage conditions are required.
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the packaging, after EXP. The expiration date is the last day of the month indicated.
Once the bottle is opened, any unused portion must be discarded after three months.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and any unused medication in the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
.
6. Package Contents and Additional Information
Composition of Risperidone Teva
The active ingredient is risperidone.
Each milliliter of Risperidone Teva oral solution contains 1 milligram of risperidone.
The other ingredients are:
Benzoic acid (E 210), 70% sorbitol solution (E 420), purified water.
Appearance of the Product and Package Contents
The oral solution is clear and colorless.
It is available in 30-milliliter and 100-milliliter bottles, both with a syringe.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Teva Pharma, S.L.U.
Anabel Segura 11, Edificio Albatros B, 1st floor.
28108 Alcobendas, Madrid, Spain
Manufacturer
Teva Czech Industries s.r.o.
Ostravská 29, 747 70,
Opava-Komárov, Czech Republic
Date of the Last Revision of this Leaflet:November 2024
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) www.aemps.gob.es.
You can access detailed and updated information about this medication by scanning the QR code included in the packaging with your smartphone. You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/69514/P_69514.html.
- Country of registration
- Average pharmacy price13.66 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RISPERIDONE TEVA 1 mg/ml ORAL SOLUTIONDosage form: TABLET, 1 mgActive substance: risperidoneManufacturer: Neuraxpharm Spain S.L.Prescription requiredDosage form: TABLET, 3 mgActive substance: risperidoneManufacturer: Neuraxpharm Spain S.L.Prescription requiredDosage form: TABLET, 6 mgActive substance: risperidoneManufacturer: Neuraxpharm Spain S.L.Prescription required
Online doctors for RISPERIDONE TEVA 1 mg/ml ORAL SOLUTION
Discuss questions about RISPERIDONE TEVA 1 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











