RIMMYRAH 10 mg/ml INJECTABLE SOLUTION

How to use RIMMYRAH 10 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Rimmyrah 10 mg/ml Solution for Injection
ranibizumab
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor, even if you think they might be unrelated to the medicine. See section 4.
Contents of the Package Leaflet
- What is Rimmyrah and what is it used for
- What you need to know before you are given Rimmyrah
- How Rimmyrah is given
- Possible side effects
- Storage of Rimmyrah
- Contents of the pack and other information
1. What is Rimmyrah and what is it used for
What is Rimmyrah
Rimmyrah is a solution that is injected into the eye. Rimmyrah belongs to a group of medicines called anti-angiogenic agents. It contains the active substance called ranibizumab.
What Rimmyrah is used for
Rimmyrah is used in adults to treat several eye diseases that cause vision problems.
These diseases are the result of damage to the retina (the light-sensitive layer at the back of the eye) caused by:
- The growth of abnormal blood vessels that leak fluid. This is seen in diseases such as age-related macular degeneration (AMD) and proliferative diabetic retinopathy (PDR, a disease caused by diabetes). It may also be associated with choroidal neovascularization (CNV) due to pathologic myopia (PM), angioid streaks, central serous chorioretinopathy, or inflammatory CNV.
- Macular edema (swelling of the center of the retina). The cause of this swelling may be diabetes (a disease known as diabetic macular edema (DME)) or a blockage of the retinal veins in the retina (a disease known as retinal vein occlusion (RVO)).
How Rimmyrah works
Rimmyrah recognizes and binds specifically to a protein called vascular endothelial growth factor A (VEGF-A) in the eyes. In excess, VEGF-A causes the growth of abnormal blood vessels and swelling in the eye that can lead to vision problems in diseases such as AMD, DME, PDR, RVO, PM, and CNV. By binding to VEGF-A, Rimmyrah can prevent it from working and prevent such abnormal growth and swelling.
In these diseases, Rimmyrah can help stabilize and, in many cases, improve your vision.
2. What you need to know before you are given Rimmyrah
Rimmyrah must not be given to you
- If you are allergic to ranibizumab or any of the other ingredients of this medicine (listed in section 6).
- If you have an eye infection or around the eye.
- If you have pain or redness (severe intraocular inflammation) in the eye.
Warnings and precautions
Talk to your doctor before you are given Rimmyrah
- Rimmyrah is given by injection into the eye. Occasionally, after treatment with Rimmyrah, an infection in the inner eye, pain or redness (inflammation), or a tear in the retina may occur. It is essential to identify and treat such an infection or retinal tear as soon as possible. Tell your doctor immediately if you notice signs such as eye pain or increased discomfort in the eye, if redness in the eye worsens, blurred vision or decreased vision, an increase in the number of small spots in your vision, or increased sensitivity to light.
- In some patients, after the injection, the pressure in the eye may increase for a short period. You may not be aware of this, so your doctor may monitor your eye pressure after each injection.
- Tell your doctor if you have had eye diseases or have received any previous treatment in the eyes, or if you have had a stroke or have had transient stroke-like symptoms (weakness or paralysis of a limb or face, difficulty speaking or understanding). This information will be taken into account to assess whether Rimmyrah is the right treatment for you.
For more detailed information on the side effects that may occur during treatment with Rimmyrah, see section 4 ("Possible side effects").
Children and adolescents (under 18 years of age)
The use of Rimmyrah in children and adolescents is not recommended, as it has not been established in these age groups.
Other medicines and Rimmyrah
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- Women who may become pregnant should use an effective method of contraception during treatment and for at least three months after the last injection of Rimmyrah.
- There is no experience with the use of Rimmyrah in pregnant women. Rimmyrah should not be used during pregnancy unless the potential benefit outweighs the potential risk to the fetus. If you are pregnant, think you may be pregnant, or plan to become pregnant, talk to your doctor before treatment with Rimmyrah.
- Small amounts of ranibizumab may pass into breast milk, so the use of Rimmyrah is not recommended during breastfeeding. Talk to your doctor or pharmacist before treatment with Rimmyrah.
Driving and using machines
After treatment with Rimmyrah, you may experience temporary blurred vision. If this happens, do not drive or use machines until this symptom disappears.
3. How Rimmyrah is given
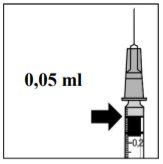
Rimmyrah is given by an ophthalmologist as a single injection into the eye under local anesthesia. The usual dose of an injection is 0.05 ml (which contains 0.5 mg of ranibizumab). The interval between two doses given in the same eye should be at least four weeks. All injections will be given by an ophthalmologist.
To prevent infection, before the injection, your doctor will carefully clean your eye. Your doctor will also give you a local anesthetic to reduce or prevent any pain you may feel with the injection.
Treatment starts with an injection of Rimmyrah every month. Your doctor will monitor the disease in your eye and, depending on how you respond to treatment, will decide whether you need further treatment and when you need to be treated.
Detailed instructions for use are given at the end of this leaflet.
Elderly patients (65 years of age and older)
Rimmyrah can be used in people 65 years of age and older, and no dose adjustment is necessary.
Before stopping treatment with Rimmyrah
If you are considering stopping treatment with Rimmyrah, go to your next appointment and discuss it with your doctor beforehand. Your doctor will advise you and decide how long you should be treated with Rimmyrah.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects associated with the use of Rimmyrah are either due to the medicine itself or the injection procedure, and most of them affect the eye.
The following are the most serious side effects:
Common serious side effects(may affect up to 1 in 10 people):
- Detachment or tear of a layer in the inner eye (retinal detachment or tear), which results in flashes of light with floating particles progressing to temporary vision loss or clouding of the lens (cataract).
Uncommon serious side effects(may affect up to 1 in 100 people):
- Blindness, infection of the eyeball (endophthalmitis) with inflammation of the inner eye.
The symptoms you may experience are eye pain or increased discomfort in the eye, if redness in the eye worsens, blurred vision or decreased vision, an increase in the number of small spots in your vision, or increased sensitivity to light.
Tell your doctor immediately if you experience any of these side effects.
The following are the most frequently reported side effects:
Very common side effects(may affect more than 1 in 10 people)
Eye side effects include:
- Eye inflammation,
- Bleeding in the back of the eye (retinal hemorrhage),
- Visual disturbances,
- Eye pain,
- Floaters (small particles or spots in the vision),
- Blood in the eye,
- Eye irritation,
- Sensation of having something in the eye,
- Increased tear production,
- Inflammation or infection of the eyelid margin,
- Dry eye,
- Redness or itching of the eye,
- Increased pressure in the eye.
Non-eye side effects include:
- Sore throat,
- Nasal congestion,
- Runny nose,
- Headache,
- Joint pain.
The following are other side effects that may occur after treatment with Rimmyrah:
Common side effects(may affect up to 1 in 10 people)
Eye side effects include:
- Decreased sharpness of vision,
- Swelling of a section of the eye (uvea, cornea),
- Inflammation of the cornea (front part of the eye),
- Small marks on the surface of the eye,
- Blurred vision,
- Bleeding at the injection site,
- Bleeding in the eye,
- Eye discharge with itching,
- Redness and swelling (conjunctivitis),
- Sensitivity to light,
- Eye discomfort,
- Swelling of the eyelid,
- Eye pain.
Non-eye side effects include:
- Urinary tract infection,
- Low red blood cell count (with symptoms such as tiredness, difficulty breathing, dizziness, paleness),
- Anxiety,
- Cough,
- Nausea,
- Allergic reactions such as rash, hives, itching, and redness of the skin.
Uncommon side effects(may affect up to 1 in 100 people)
Eye side effects include:
- Inflammation and bleeding in the front part of the eye,
- Accumulation of pus in the eye,
- Changes in the central part of the eye surface,
- Pain or irritation at the injection site,
- Abnormal sensation in the eye,
- Irritation of the eyelid.
Reporting of side effects
If you experience any side effects, talk to your doctor, even if you think they might be unrelated to the medicine. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Rimmyrah
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after CAD and on the label of the vial after EXP. The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2°C and 8°C). Do not freeze.
- Before use, the unopened vial can be stored at room temperature (25°C) for a maximum of 24 hours.
- Keep the vial in the outer packaging to protect it from light.
- Do not use any damaged packaging.
6. Container Contents and Additional Information
Rimmyrah Composition
- The active ingredient is ranibizumab. Each ml contains 10 mg of ranibizumab. Each vial contains 2.3 mg of ranibizumab in 0.23 ml of solution. This provides a sufficient amount to deliver a single dose of 0.05 ml, which contains 0.5 mg of ranibizumab.
- The other components are trehalose dihydrate, histidine hydrochloride monohydrate, histidine, polysorbate 20 (E432), and water for injectable preparations.
Product Appearance and Container Contents
Rimmyrah is an injectable solution contained in a vial (0.23 ml). The solution is transparent to slightly opalescent, colorless to brownish, and aqueous.
There are two types of packaging available:
Container with only vial
A container containing a glass vial with ranibizumab, with a chlorobutyl rubber stopper. The vial is for single use.
Container with vial and needle with filter
A container containing a glass vial with ranibizumab, with a chlorobutyl rubber stopper and a blunt needle with filter (18G x 1½ inches, 1.2 mm x 40 mm, 5 microns) for extracting the vial contents. All components are for single use.
Marketing Authorization Holder
QILU PHARMA SPAIN S.L.
Paseo de la Castellana 40, 8th floor
28046 Madrid
Spain
Manufacturer
KYMOS, S.L.
Ronda De Can Fatjo 7 B
Parc Tecnologic Del Valles
Cerdanyola Del Valles
Barcelona
08290
Spain
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Lithuania Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Luxembourg/Luxemburg Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Czech Republic Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Hungary Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Denmark Orion Pharma A/S Tel: +45 8614 00 00 | Malta Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Germany Orion Pharma GmbH Tel: +49 40 899 689-0 | Netherlands Orion Pharma BV/SRL Tel: +32 (0)15 64 10 20 |
Estonia Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Norway Orion Pharma AS Tel: +47 4000 42 10 |
Greece Orion Pharma Hellas M.E.P.E. Tel: +30 210 980 3355 | Austria Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Spain Orion Pharma S.L. Tel: +34 915 99 86 01 | Poland Orion Pharma Poland Sp. z o.o. Tel: +48 22 833 31 77 |
France Orion Pharma Tel: +33 (0)1 85 18 00 00 | Portugal Orionfin Unipessoal Lda. Tel: +351 211 546 820 |
Croatia Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Ireland Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Romania Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Slovenia Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Iceland Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Slovakia Qilu Pharma Spain S.L. Tel: +34 911 841 918 |
Italy Orion Pharma S.r.l. Tel: +39 02 67876111 | Finland Orion Pharma Tel: +358 10 4261 |
Cyprus Qilu Pharma Spain S.L. Tel: +34 911 841 918 | Sweden Orion Pharma AB Tel: +46 8 623 6440 |
Latvia Qilu Pharma Spain S.L. Tel: +34 911 841 918 | United Kingdom (Northern Ireland) Orion Pharma (Ireland) Limited Tel: +353 1 428 7777 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
THIS INFORMATION IS INTENDED ONLY FOR HEALTHCARE PROFESSIONALS:
See also section 3 "How to administer Rimmyrah".
Single-use vial. For intravitreal use only.
Rimmyrah should be administered by an ophthalmologist experienced in administering intravitreal injections.
In exudative age-related macular degeneration (AMD), neovascular age-related macular degeneration (NVC), retinal pigment epithelial detachment (RDP), and visual impairment due to macular edema (EMD) or macular edema secondary to branch retinal vein occlusion (BRVO), the recommended dose of Rimmyrah is 0.5 mg administered as a single intravitreal injection. This corresponds to an injection volume of 0.05 ml. The interval between two doses injected into the same eye should be at least four weeks.
Treatment is initiated with one injection per month until maximum visual acuity is achieved and/or there are no signs of disease activity, i.e., no change in visual acuity or other signs and symptoms of the disease under continued treatment. In patients with exudative AMD, EMD, RDP, and BRVO, three or more consecutive monthly injections may be necessary initially.
From that point on, monitoring and treatment intervals should be determined based on medical judgment and the activity of the disease, assessed by visual acuity and/or anatomical parameters.
Treatment with Rimmyrah should be discontinued if, in the physician's judgment, visual and anatomical parameters indicate that the patient is not benefiting from continued treatment.
Monitoring to determine disease activity may include clinical examination, functional testing, or imaging techniques (e.g., optical coherence tomography or fluorescein angiography).
If patients are being treated according to a treat-and-extend regimen, once maximum visual acuity is achieved and/or there are no signs of disease activity, treatment intervals can be gradually extended until signs of disease activity or visual impairment recur. In the case of exudative AMD, the treatment interval should not be extended by more than two weeks at a time, and in the case of EMD, it can be extended up to one month at a time. For RDP and BRVO, treatment intervals can also be gradually extended; however, available data are insufficient to determine the duration of these intervals. If disease activity recurs, the treatment interval should be shortened accordingly.
Treatment of visual impairment due to NVC should be determined individually for each patient based on disease activity. Some patients may require only one injection during the first 12 months; others may require more frequent treatment, including monthly injections. In the case of NVC secondary to pathologic myopia (PM), many patients may require only one or two injections during the first year.
Ranibizumab and Laser Photocoagulation in EMD and Macular Edema Secondary to Branch Retinal Vein Occlusion (BRVO)
There is some experience with ranibizumab administered concomitantly with laser photocoagulation. When administered on the same day, ranibizumab should be administered at least 30 minutes after laser photocoagulation. Ranibizumab can be administered in patients who have received prior laser photocoagulation.
Ranibizumab and Photodynamic Therapy with Verteporfin in NVC Secondary to Pathologic Myopia (PM)
There is no experience with the concomitant administration of ranibizumab and verteporfin.
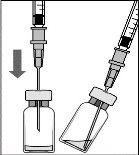
Before administering Rimmyrah, the medicinal product should be inspected visually to ensure the absence of particles, discoloration, or alteration. If particles, discoloration, or alteration are observed, the vial should be discarded in accordance with local disposal regulations.
The injection procedure should be carried out under aseptic conditions, including surgical hand washing, use of sterile gloves, a sterile field, a sterile blepharostat (or equivalent), and the availability of a sterile paracentesis (if necessary). Before performing the intravitreal injection procedure, the patient's medical history should be thoroughly evaluated for hypersensitivity reactions. Before injection, adequate anesthesia and a broad-spectrum topical microbicide should be administered to disinfect the periocular skin, eyelid, and ocular surface, in accordance with local practice.
Container with only vial
The vial is for single use. After injection, any unused product should be discarded. No vial showing signs of damage or tampering should be used. Sterility can only be guaranteed if the container seal remains intact.
For preparation and intravitreal injection, the following medical devices (for single use) are required:
- a 5-micron filter needle (18G x 1½ inches, 1.2 mm x 40 mm)
- a 1-ml sterile syringe (including a 0.05-ml mark)
- an injection needle (30G x ½ inch). These medical devices are not included in the Rimmyrah packaging.
Container with vial and needle with filter
All components are sterile and for single use. No component with a damaged or tampered packaging should be used. Sterility can only be guaranteed if the packaging seal of the components remains intact. Reuse may lead to infection or other disease/injury.
For preparation and intravitreal injection, the following medical devices (for single use) are required:
- a 5-micron filter needle (18G x 1½ inches, 1.2 mm x 40 mm, supplied)
- a 1-ml sterile syringe (including a 0.05-ml mark, not included with the Rimmyrah packaging)
- an injection needle (30G x ½ inch, not included with the Rimmyrah packaging)
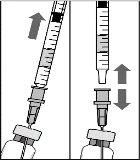
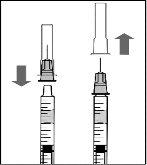
To prepare Rimmyrah for intravitreal administration to adult patients, follow these instructions:
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered product should be clearly recorded.
|
|
|
Note: Hold the injection needle by the hub while removing the needle cap.
Note: Do not wipe the injection needle. Do not pull the plunger back. |
The injection needle should be inserted 3.5 to 4.0 mm behind the limbus into the vitreous cavity, avoiding the horizontal meridian and with the needle directed towards the center of the eyeball. The injection volume of 0.05 ml should then be delivered. A different scleral location should be used for subsequent injections.
After injection, do not cover the needle with the needle cap or separate it from the syringe. Dispose of the used syringe and needle in a puncture-resistant container or according to local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RIMMYRAH 10 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Samsung Bioepis Nl B.V.Prescription requiredDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for RIMMYRAH 10 mg/ml INJECTABLE SOLUTION
Discuss questions about RIMMYRAH 10 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions