RILAST 160/4.5 micrograms/inhalation pressurized suspension for inhalation

How to use RILAST 160/4.5 micrograms/inhalation pressurized suspension for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Rilast 160 micrograms/4.5 micrograms/inhalation inhalation suspension in pressurized container
Budesonide/formoterol fumarate dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Rilast and what is it used for
- What you need to know before you use Rilast
- How to use Rilast
- Possible side effects
- Storing Rilast
- Contents of the pack and other information
1. What is Rilast and what is it used for
Rilast is an inhaler used for the symptomatic treatment of Chronic Obstructive Pulmonary Disease (COPD) in adults over 18 years of age. COPD is a chronic disease of the lung airways, often caused by tobacco smoke. It contains two different medicines: budesonide and formoterol fumarate dihydrate.
- Budesonide belongs to a group of medicines called "corticosteroids", and acts by reducing and preventing lung inflammation.
- Formoterol fumarate dihydrate belongs to a group of medicines called "long-acting beta2 adrenergic agonists" or "bronchodilators", and acts by relaxing the muscles of the airways, making it easier to breathe.
Do not use this medicine as a "reliever" inhaler.
2. What you need to know before you use Rilast
Do not use Rilast:
- if you are allergic to budesonide, formoterol or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Rilast if:
- You have diabetes.
- You have a lung infection.
- You have high blood pressure, or have ever had any heart disease (including irregular heartbeats, rapid pulse, narrowing of the arteries or heart failure).
- You have thyroid or adrenal gland problems.
- You have low potassium levels in your blood.
- You have severe liver problems.
Contact your doctor if you experience blurred vision or other visual disturbances.
Athletes
This medicine contains formoterol, which may produce a positive result in doping tests.
Children and adolescents
Rilast is not recommended for children and adolescents under 18 years of age.
Other medicines and Rilast
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
In particular, tell your doctor or pharmacist if you are using any of the following medicines:
- Beta-blocker medicines (such as atenolol and propranolol for high blood pressure), including eye drops (such as timolol for glaucoma).
- Medicines to treat fast or irregular heartbeats (such as quinidine).
- Medicines such as digoxin, used to treat heart failure.
- Diuretics (such as furosemide), used to treat high blood pressure.
- Oral steroid medicines (such as prednisolone).
- Xanthine medicines (such as theophylline or aminophylline), commonly used to treat COPD or asthma.
- Other bronchodilators (such as salbutamol).
- Tricyclic antidepressants (such as amitriptyline) and the antidepressant nefazodone.
- Phenothiazine medicines (such as chlorpromazine and prochlorperazine).
- Medicines called "HIV protease inhibitors" (such as ritonavir) for the treatment of HIV.
- Medicines for treating infections (such as ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin and telithromycin).
- Medicines for Parkinson's disease (such as levodopa).
- Medicines for thyroid problems (such as levothyroxine).
If you are in any of these situations, or if you are not sure, ask your doctor or pharmacist before using Rilast.
Also, inform your doctor or pharmacist if you are going to have a general anesthetic for a surgical operation or for dental treatment.
Pregnancy, breastfeeding and fertility
- Tell your doctor before using this medicine if you are pregnant, or planning to become pregnant; do not use Rilast unless your doctor tells you to.
- If you become pregnant while using Rilast, do not stop using it and consult your doctor immediately.
- If you are breastfeeding, talk to your doctor before using Rilast.
Driving and using machines
Rilast has no or negligible influence on the ability to drive and use machines.
3. How to use Rilast
- Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
- It is important to use Rilast every day, even if you do not have symptoms of COPD at that time.
The recommended dose is 2 inhalations twice a day. Rilast is not recommended for children and adolescents under 18 years of age.
If you have been taking oral steroid tablets for COPD, your doctor may reduce the number of tablets you take once you start treatment with Rilast. If you have been taking oral steroid tablets for a long time, your doctor may want to do a blood test from time to time. When the number of oral steroid tablets is reduced, you may feel unwell, even if your lung symptoms are improving. In this case, some symptoms such as nasal congestion or runny nose, weakness or pain in the muscles or joints, and skin rash (hives) may appear temporarily. Contact your doctor if any of these symptoms worry you, or if you have any other symptoms such as headache, tiredness, nausea or vomiting. If allergy or arthritis symptoms appear, you may need to take another medicine. You should consult your doctor if you are concerned about whether you should continue using Rilast.
Your doctor may consider adding oral steroid tablets to your usual treatment during periods of stress (for example, when you have a chest infection or before a surgical operation).
Important information about COPD symptoms
If while using Rilast you feel difficulty breathing or wheezing, you should continue using it and contact your doctor as soon as possible, as you may need additional treatment.
Contact your doctor immediately if:
- Your breathing worsens or you wake up often at night with symptoms of shortness of breath.
- You start to feel chest tightness in the morning, or chest tightness lasts longer than usual.
- These signs may indicate that your COPD is not well controlled and you may need immediate different or additional treatment.
It is possible that your doctor will prescribe other bronchodilators, such as anticholinergics (such as tiotropium or ipratropium bromide), for your COPD.
Information about your new Rilast inhaler
- Before using your new Rilast inhaler, take it out of the aluminum foil wrapper. Throw away the wrapper and the drying agent that is inside the wrapper. Do not use the inhaler if the drying agent has come out of the package.
- The inhaler must be used within 3 months of taking it out of the aluminum foil wrapper. Write the expiration date (3 months from the date you took it out of the wrapper) on the inhaler label to remind you when to stop using the inhaler.
- The image shows the parts of the inhaler, which will already be assembled when you receive it. Do not separate the parts. If the container becomes loose, put it back in the inhaler and continue using it.
Preparing Rilast
The inhaler needs to be prepared for use in the following situations:
- If you are using your new Rilast for the first time.
- If you have not used it for more than 7 days.
- If it has been dropped.
To prepare the inhaler for use, follow these instructions:
- Shake the inhaler well for at least 5 seconds to mix the contents of the aerosol cartridge.
- Remove the mouthpiece cap by pressing gently on the protrusions on the side. The cap strap will remain attached to the inhaler.
- Hold the inhaler upright. Then, press the counter (on the top of the inhaler) to release a spray into the air. You can use one or both hands, as shown in the photos.
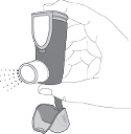
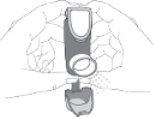
- Remove your finger(s) from the counter.
- Wait 10 seconds, shake well and repeat steps 3 and 4.
- Your inhaler is now ready for use.
How to take an inhalation
Each time you need to take an inhalation, follow these instructions:
- Shake the inhaler well for at least 5 seconds to mix the contents of the aerosol cartridge.
- Remove the mouthpiece cap by pressing gently on the protrusions on the side. Check that the mouthpiece is not blocked.
- Hold the inhaler upright (you can use one or both hands). Breathe out gently.
- Gently place the mouthpiece between your teeth. Close your lips.
- Breathe in slowly and deeply through your mouth. Press the counter (on the top of the inhaler) firmly to release a spray. Hold your breath for a moment while pressing the counter. Breathe in at the same time as you press the counter to ensure that the medicine reaches your lungs.
- Hold your breath for 10 seconds, or as long as is comfortable.
- Before breathing out, release your finger from the counter and remove the inhaler from your mouth. Keep the inhaler upright.
 Breathe out gently. Before taking another inhalation, shake the inhaler well for at least 5 seconds and repeat steps 3 to 7.
Breathe out gently. Before taking another inhalation, shake the inhaler well for at least 5 seconds and repeat steps 3 to 7.- Put the mouthpiece cap back on.
- Rinse your mouth with water after the morning and evening doses. Do not swallow.
Using a spacer
Your doctor, nurse or pharmacist may suggest using a spacer (such as Aerochamber Plus Flow Vuor Aerochamber Plus). Follow the instructions that come with the spacer.
Cleaning Rilast
- Clean the inside and outside of the mouthpiece at least once a week with a dry cloth.
- Do not use water or liquids and do not separate the cartridge from the inhaler.
How do I know when I need to replace my Rilast inhaler?
- The counter on the top tells you how many inhalations are left in your Rilast inhaler. It starts with 120 inhalations when it is full.
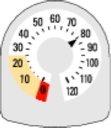
- Each time you take an inhalation, or release a spray into the air, the needle starts counting down towards zero ('0').
- When the needle first enters the yellow area, it means that there are about 20 inhalations left.
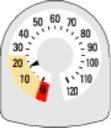
- When the needle reaches '0', you must stop using your new Rilast inhaler. You may not feel that the inhaler is empty and it may seem to be working, but you will not receive the correct amount of medicine if you continue to use it.
If you use more Rilast than you should
If you have used more Rilast than you should, contact your doctor or pharmacist. The most common symptoms and signs that may occur after an overdose are tremors, headache and rapid heartbeat.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone: 91 5620420, indicating the medicine and the amount taken.
If you forget to use Rilast
- If you forget to take a dose of Rilast, follow the recommended treatment as soon as you remember. However, if it is almost time for your next dose, do not worry about the missed dose.
- Do nottake a double dose to make up for missed doses.
If you stop using Rilast
Before stopping use of Rilast, you should consult your doctor or pharmacist. If you stop using Rilast, the symptoms and signs of COPD may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following, stop using Rilast and consult your doctor immediately:
- Your face, particularly around the mouth (tongue and/or throat), swells, or you experience hives, along with difficulty breathing (angioedema) and/or a sudden feeling of fainting, which indicates that you may be having an allergic reaction. This occurs rarely, affecting less than 1 in 1,000 patients.
- You experience wheezing or difficulty breathing immediately after using your inhaler. If you experience any of these symptoms, stop using Rilast immediately and use your "reliever" inhaler. Contact your doctor immediately, as you may need to change your treatment.This occurs very rarely, affecting less than 1 in 10,000 patients.
Other possible side effects:
Common (may affect up to 1 in 10 patients)
- Palpitations (feeling your heartbeat), tremors or shivers. These effects, if they occur, are usually mild and disappear as you continue to use Rilast.
- Thrush (fungal infection) in the mouth; this effect is less likely if you rinse your mouth with water after using Rilast.
- Mild irritation of the throat, cough, hoarseness.
- Headache.
- Pneumonia (lung infection) in patients with COPD.
Tell your doctor if you experience any of the following symptoms while inhaling Rilast, they could be symptoms of a lung infection:
- Fever or chills.
- Increased production of mucus, change in the color of the mucus.
- Increased cough or increased difficulty breathing.
Uncommon (may affect up to 1 in 100 patients)
- Agitation, restlessness, nervousness.
- Difficulty sleeping.
- Dizziness.
- Nausea (discomfort).
- Rapid heartbeat.
- Bruises on the skin.
- Muscle cramps.
- Blurred vision.
Rare (may affect up to 1 in 1,000 patients)
- Rash, itching.
- Bronchospasm (contraction of the airway muscles, causing wheezing). If wheezing occurs suddenly just after using Rilast, stop using it and consult your doctor immediately.
- Low potassium levels in the blood.
- Irregular heartbeat.
Very rare (may affect up to 1 in 10,000 patients)
- Depression.
- Changes in behavior, especially in children.
- Chest pain or tightness (angina pectoris).
- Increased blood sugar (glucose) levels.
- Changes in taste, such as unpleasant mouth taste.
- Changes in blood pressure.
Inhaled corticosteroids may affect the normal production of steroid hormones in the body, especially if high doses are used for a long time. These effects include:
- changes in bone mineral density (thinning of the bones)
- cataracts (loss of transparency of the lens in the eye)
- glaucoma (increased eye pressure)
- growth retardation in children and adolescents
- effects on the adrenal glands (small glands located next to the kidneys).
These effects are much less likely with inhaled corticosteroids than with oral corticosteroid tablets.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Rilast
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton, the aluminum foil, or the label after CAD. The expiry date is the last day of the month indicated.
- As with most pressurized inhalation medications, the effect of this medicine may decrease when the container is cold. For best results, this medicine should be at room temperature before use. Do not refrigerate or freeze. Protect from frost and direct sunlight.
- It should be used within 3 months after the inhaler has been removed from its aluminum foil wrapper. Write the expiry date (3 months from the opening of the wrapper) on the inhaler label to remind you when to stop using the inhaler.
- Always replace the mouthpiece cap and click it into position after using the inhaler.
- Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need at the pharmacy's SIGRE point. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
Warning: The cartridge contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not pierce the container. The cartridge should not be broken, punctured, or burned, even when it appears to be empty.
6. Package Contents and Additional Information
Composition of Rilast
The active ingredients are budesonide and formoterol fumarate dihydrate. Each inhaled dose contains 160 micrograms of budesonide and 4.5 micrograms of formoterol fumarate dihydrate.
The other excipients are apaflurane (HFA 227), povidone, and Macrogol. This inhaler does not contain CFCs.
This medicine contains fluorinated greenhouse gases. Each inhaler contains 10.6 g of apaflurane (HFC-227ea) which corresponds to 0.034 tons of CO2 equivalent (global warming potential GWP = 3,220).
Appearance of the Product and Package Contents
Rilast is an inhaler that contains your medicine. The pressure cartridge with an attached dose indicator contains a white suspension for inhalation. The cartridge is mounted in a red plastic adapter with a white plastic mouthpiece and an integrated gray plastic cap. Each inhaler contains 120 inhalations after it has been prepared for use. Each inhaler is individually packaged in an aluminum paper wrapper containing a desiccant.
Rilast 160 micrograms/4.5 micrograms/inhalation suspension for inhalation in a pressurized container (budesonide/formoterol fumarate dihydrate) is available in packages with a single inhaler.
Marketing Authorization Holder and Manufacturer
The holder is:
Laboratory TAU, S.A.
C/ Puerto de Somport 21-23
28050 Madrid
Spain
The manufacturer is:
AstraZeneca Dunkerque Production
224, Avenue de la Dordogne BP 41
59640 Dunkerque (France)
This medicine is authorized in the member states of the European Economic Area under the following names:
Country | Trade Name and Concentration |
Spain | Rilast 160 μg/4.5 μg/inhalation suspension for inhalation in a pressurized container |
Sweden | Gardette 160 micrograms/4.5 micrograms/inhalation |
Date of the Last Revision of this Leaflet: January 2025
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price39.56 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RILAST 160/4.5 micrograms/inhalation pressurized suspension for inhalationDosage form: PULMONARY INHALATION, 160 micrograms / 4.5 microgramsActive substance: formoterol and budesonideManufacturer: Teva Pharma B.V.Prescription requiredDosage form: PULMONARY INHALATION, 320 micrograms / 9 microgramsActive substance: formoterol and budesonideManufacturer: Teva Pharma B.V.Prescription requiredDosage form: PULMONARY INHALATION, 160 MICROGRAMS/4.5 MICROGRAMSActive substance: formoterol and budesonideManufacturer: Cipla Europe N.V.Prescription required
Online doctors for RILAST 160/4.5 micrograms/inhalation pressurized suspension for inhalation
Discuss questions about RILAST 160/4.5 micrograms/inhalation pressurized suspension for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions