REBIF 22 micrograms/0.5 ml Injectable Solution in Cartridge

How to use REBIF 22 micrograms/0.5 ml Injectable Solution in Cartridge
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Rebif 22micrograms/0.5ml solution for injection in a cartridge
interferon beta-1a
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Rebif and what is it used for
- What you need to know before you use Rebif
- How to use Rebif
- Possible side effects
- Storage of Rebif
- Contents of the pack and other information
1. What is Rebif and what is it used for
Rebif belongs to a class of medicines known as interferons. They are natural substances that transmit messages between cells. Interferons are produced by the body and play an essential role in the immune system. Through mechanisms that are not fully understood, interferons help to limit the damage to the central nervous system associated with multiple sclerosis.
Rebif is a highly purified soluble protein, which is similar to the natural interferon beta produced by the human body.
Rebif is used to treat multiple sclerosis. It has been shown to reduce the number and severity of relapses and to delay the progression of disability.
2. What you need to know before you use Rebif
Do not use Rebif
- If you are allergic to natural or recombinant interferon beta or to any of the other ingredients of this medicine (listed in section 6).
- If you currently have severe depression.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Rebif.
- Rebif should only be used under the supervision of your doctor.
- Before starting treatment with Rebif, read carefully and follow the recommendations given in the section "How to use Rebif" to minimize the risk of necrosis at the injection site (skin breakdown and tissue destruction) that has been reported in patients treated with Rebif. If you notice any unpleasant local reactions, contact your doctor.
- Tell your doctor or pharmacist before you start taking Rebif if you have an allergy (hypersensitivity) to any other medicine.
- During treatment, blood clots may form in small blood vessels. These clots could affect your kidneys. This can occur after several weeks or several years after starting treatment with Rebif. Your doctor may want to check your blood pressure, blood (platelet count), and kidney function.
Tell your doctor if you have any disease of
- the bone marrow,
- kidney,
- liver,
- heart,
- thyroid,
- or if you have had depression,
- or if you have a history of epileptic seizures,
so that your doctor can closely monitor your treatment and any worsening of these diseases.
Other medicines and Rebif
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
In particular, you should tell your doctor if you are using antiepileptics or antidepressants.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
No harmful effects on breastfed babies/infants are expected. Rebif can be used during breastfeeding.
Driving and using machines
The effects of your disease or its treatment may affect your ability to drive or use machines. If this affects you, discuss it with your doctor.
Rebif contains sodium and benzyl alcohol
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
This medicine contains 2.5 mg of benzyl alcohol per dose. Benzyl alcohol may cause allergic reactions.
Benzyl alcohol has been linked to the risk of serious side effects, including breathing problems ("gasping syndrome") in children.
This product should not be used for more than one week in children under 3 years of age unless advised by your doctor or pharmacist.
Consult your doctor or pharmacist if you are pregnant or breastfeeding or if you have liver or kidney disease. This is because large amounts of benzyl alcohol can build up in your body and cause side effects (metabolic acidosis).
3. How to use Rebif
This medicine is for multidose use.
Follow exactly the administration instructions for this medicine given by your doctor. If you are unsure, consult your doctor again.
Dose
The usual dose is 44 micrograms (12 million IU) administered three times a week. Your doctor has prescribed a lower dose of 22 micrograms (6 million IU), also administered three times a week. This lower dose is recommended for patients who cannot tolerate the higher dose.
Rebif should be administered three times a week and, if possible:
- on the same three days of each week (at least 48 hours apart; for example, Monday, Wednesday, and Friday);
- at the same time of day (preferably in the afternoon).
Use in children and adolescents (2 to 17 years)
No formal clinical trials have been conducted in children or adolescents. However, clinical data are available that suggest the safety profile in children and adolescents given Rebif 22 micrograms or Rebif 44 micrograms three times a week is similar to that observed in adults.
Use in children (under 2 years of age)
Rebif is not recommended for use in children under 2 years of age.
Method of administration
- Rebif should be injected subcutaneously (under the skin).
- The first injection(s) should be administered under the supervision of a qualified healthcare professional. After receiving proper training, you, a family member, friend, or caregiver can use Rebif cartridges with the RebiSmart device to administer the medicine at home.
- The cartridge should be used with the RebiSmart electronic injection device.
- Complete instructions for use are included with the device; follow them carefully.
- The following are brief instructions on how to use the Rebif cartridges.
Before you start
- Wash your hands thoroughly with soap and water.
- Remove the Rebif cartridge from its blister pack, removing the plastic cover.
- Check (immediately after removing from the refrigerator) that the cartridge has not been accidentally frozen in the packaging or inside the device. Only use clear to opalescent solutions without particles and without visible signs of deterioration.
- To place the cartridge in the device and perform the injection, follow the instructions for use (User Manual) provided with your device.
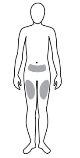
Where to inject Rebif
|
|
How to inject Rebif
- Your doctor will tell you how to choose the correct dose of 22 micrograms. Also, read the instructions in the manual provided with the device (RebiSmart).
RebiSmart |
|
After injecting Rebif with RebiSmart
- Remove and dispose of the needle according to the instructions for use provided with your device.
- Gently massage the injection site with a cotton ball or dry swab.
- Store the device containing a Rebif cartridge as indicated in section 5 "Storage of Rebif".
If you have any doubts, ask your doctor, nurse, or pharmacist.
If you use more Rebif than you should
In case of overdose, contact your doctor immediately.
If you forget to use Rebif
If you miss an injection, continue injecting from the day you are due for the next dose. Do not use a double dose to make up for forgotten doses.
If you stop using Rebif
The effects of Rebif may not be noticeable immediately. Therefore, you should not stop using Rebif without talking to your doctor first.
Do not stop treatment without first discussing it with your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Tell your doctor immediately and interrupt treatment with Rebif if you experience any of the following adverse effects:
- Severe allergic reactions (hypersensitivity).Contact your doctor immediately or seekurgent medical attention if, immediately after administration of Rebif, you experience difficulty breathing that may be accompanied by swelling of the face, lips, tongue, and throat, hives, itching all over the body, and a feeling of weakness and fatigue. These reactions are rare(may affect up to 1 in 1,000 people).
- Tell your doctor immediately if you experience any of the following possible symptoms of a liver problem: jaundice (yellowing of the skin or the white parts of the eyes), generalized itching, loss of appetite accompanied by nausea and vomiting, and ease of bruising. Severe liver problems may be associated with other symptoms such as difficulty concentrating, drowsiness, and confusion.
- Depressionis frequent(may affect up to 1 in 10 people) in patients treated for multiple sclerosis. If you feel depressed or experience suicidal thoughts, inform your doctor immediately.
Consult your doctor if you experience any of the following adverse effects:
- Flu-like symptoms,such as headache, fever, chills, muscle and joint pain, fatigue, and nausea are very frequent(may affect more than 1 in 10 people).
These symptoms are normally mild and more frequent at the start of treatment, decreasing with continued use.
To help reduce these symptoms, your doctor may recommend administering a pain reliever before each dose of Rebif and at 24 hours after each injection.
- Injection site reactions,including redness, swelling, discoloration, inflammation, pain, and skin fragility are very frequent.
The appearance of injection site reactions generally decreases over time.
Tissue destruction (necrosis), abscess formation, and lump formation at the injection site are uncommon(may affect up to 1 in 100 people).
See the recommendations in "Warnings and Precautions" to minimize the risk of injection site reactions.
The injection site may become infected (uncommon); the skin may become inflamed, painful, and hardened, and the area may be very painful. If you experience any of these symptoms, contact your doctor.
- Some test resultsmay be altered. These changes are generally not perceived by the patient (no symptoms), are usually reversible and mild, and many of them do not require specific treatment.
The number of red blood cells, white blood cells, or platelets may decrease individually (very frequent)or all at the same time (rare). The possible symptoms associated with these changes may include fatigue, decreased ability to react to infections, bruising, or bleeding of unknown cause. Liver function may be altered (very frequent). Cases of liver inflammation (hepatitis) have been reported (uncommon). If you experience any symptoms suggesting a liver disorder, such as loss of appetite accompanied by other symptoms such as nausea, vomiting, jaundice, contact your doctor immediately (see above "Tell your doctor immediately").
- Thyroid dysfunctionis uncommon. The thyroid gland may function excessively or insufficiently. These changes in thyroid activity are not perceived by patients as symptoms of a disorder, but your doctor may recommend an analysis if deemed appropriate.
- Pseudo-relapse (or false relapse) of multiple sclerosis(frequency not known): it is possible that at the start of treatment with Rebif, you may experience symptoms similar to a relapse of multiple sclerosis. For example, you may notice your muscles tense or very weak, preventing free movement. In some cases, these symptoms are associated with fever or flu-like symptoms as described above. If you notice any of these adverse effects, consult your doctor.
Other possible adverse effects may include:
Very frequent (may affect more than 1 in 10 people):
- Headache.
Frequent (may affect up to 1 in 10 people):
- Insomnia (difficulty sleeping)
- Diarrhea, nausea, vomiting
- Jaundice, rashes (skin eruptions)
- Muscle and joint pain
- Fatigue, fever, and chills
- Hair loss.
Uncommon (may affect up to 1 in 100 people):
- Hives
- Seizures
- Liver inflammation (hepatitis)
- Breathing difficulties
- Blood clots such as deep vein thrombosis
- Retinal alterations (eye fundus) such as inflammation, blood clots with consequent vision disorders (vision changes, vision loss)
- Increased sweating.
Rare (may affect up to 1 in 1,000 people):
- Suicidal attempt
- Severe skin reactions, some with mucosal damage
- Blood clots in small blood vessels that can affect your kidneys (thrombotic thrombocytopenic purpura or hemolytic uremic syndrome). Symptoms may include increased bruising, bleeding, fever, extreme weakness, headache, dizziness, or fainting. Your doctor may find alterations in your blood and kidney function.
- Drug-induced lupus erythematosus: a long-term adverse effect of using Rebif. Symptoms may include muscle pain, joint pain and swelling, and rashes. You may also experience other signs such as fever, weight loss, and fatigue. Symptoms usually disappear within one or two weeks after treatment discontinuation.
- Kidney problems, including scarring that can reduce kidney function.
If you experience any of these symptoms or all of them:
- Foamy urine
- Fatigue
- Swelling, especially of ankles and eyelids, and weight gain.
Tell your doctor, as they may be signs of a possible kidney problem.
The following adverse effects were reported for interferon beta (frequency not known)
- Dizziness
- Nervousness
- Loss of appetite
- Vasodilation and palpitations
- Irregularities and/or changes in menstrual flow.
- Pulmonary arterial hypertension is a disease in which there is a significant narrowing of the blood vessels in the lungs, causing an increase in pressure in the blood vessels that carry blood from the heart to the lungs. Pulmonary arterial hypertension was reported at various times, even several years after starting treatment with Rebif.
- Inflammation of the fatty tissue under the skin (panniculitis), which can be noticed as hardened and may appear as lumps or red and painful spots.
Do not interrupt or change treatment without your doctor's advice.
Children and Adolescents
Adverse effects in children and adolescents are similar to those observed in adults.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Rebif
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date stated on the label after CAD.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze. (To avoid accidental freezing, do not place near the freezer).
After the first injection, use within the next 28 days.
The device (RebiSmart) with a Rebif cartridge inside should be stored in its protective case in a refrigerator (between 2°C and 8°C). For ambulatory use, you can remove Rebif from the refrigerator and store it at a temperature not exceeding 25°C for a single maximum period of 14 days. After that, Rebif must be returned to the refrigerator and used before the expiration date.
Store in the original packaging to protect it from light.
Do not use this medicine if you observe any visible signs of deterioration, such as if the solution in the cartridge is no longer clear and colorless or if it contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Rebif
- The active ingredient is interferon beta-1a. Each cartridge contains 66 micrograms corresponding to 18 million International Units (IU) of interferon beta-1a.
- The other components are mannitol, poloxamer 188, L-methionine, benzyl alcohol, sodium acetate, acetic acid, sodium hydroxide, and water for injectable preparations.
Appearance of the Product and Package Contents
Pre-filled syringe (type 1 glass) with a stopper (rubber) and a closure cap (aluminum with halobutyl rubber) containing 1.5 ml of solution. Package size of 4 or 12 syringes. Only some package sizes may be marketed.
The cartridge must be used with the RebiSmart electronic injection device. The device is supplied separately.
Marketing Authorization Holder
Merck Europe B.V.
Gustav Mahlerplein 102
1082 MA Amsterdam
Netherlands
Manufacturer
Merck Serono S.p.A.
Via delle Magnolie 15
I-70026 Modugno (Bari)
Italy
Date of Last Revision of this Leaflet: 01/2023
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REBIF 22 micrograms/0.5 ml Injectable Solution in CartridgeDosage form: INJECTABLE, 30 µgActive substance: interferon beta-1aManufacturer: Biogen Netherlands B.V.Prescription requiredDosage form: INJECTABLE, 30 µgActive substance: interferon beta-1aManufacturer: Biogen Netherlands B.V.Prescription requiredDosage form: INJECTABLE, 44 µgActive substance: interferon beta-1aManufacturer: Merck Europe B.V.Prescription required
Online doctors for REBIF 22 micrograms/0.5 ml Injectable Solution in Cartridge
Discuss questions about REBIF 22 micrograms/0.5 ml Injectable Solution in Cartridge, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions