RANIVISIO 10 mg/ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE

How to use RANIVISIO 10 mg/ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ranivisio 10 mg/ml Solution for Injection in Pre-filled Syringe
ranibizumab
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor, even if they are not listed in this leaflet. See section 4.
Contents of the Pack
- What is Ranivisio and what is it used for
- What you need to know before you are given Ranivisio
- How Ranivisio is given
- Possible side effects
- Storing Ranivisio
- Contents of the pack and other information
1. What is Ranivisio and what is it used for
What is Ranivisio
Ranivisio is a solution that is injected into the eye. Ranivisio belongs to a group of medicines called anti-angiogenic agents. It contains the active substance called ranibizumab.
What Ranivisio is used for
Ranivisio is used in adults to treat several eye diseases that cause vision problems.
These diseases are the result of damage to the retina (the light-sensitive layer at the back of the eye) caused by:
- The growth of abnormal blood vessels that leak fluid. This is seen in diseases such as age-related macular degeneration (AMD) and proliferative diabetic retinopathy (PDR, a disease caused by diabetes). It may also be associated with choroidal neovascularization (CNV) due to pathologic myopia (PM), angioid streaks, central serous chorioretinopathy, or inflammatory CNV.
- Macular edema (swelling of the center of the retina). The cause of this swelling may be diabetes (a disease known as diabetic macular edema, DME) or a blockage of the retinal veins in the retina (a disease known as retinal vein occlusion, RVO).
How Ranivisio works
Ranivisio recognizes and binds specifically to a protein called vascular endothelial growth factor A (VEGF-A) present in the eyes. In excess, VEGF-A causes the growth of abnormal blood vessels and swelling in the eye that can lead to vision problems in diseases such as AMD, DME, PDR, RVO, PM, and CNV.
By binding to VEGF-A, Ranivisio can prevent it from working and prevent such abnormal growth and swelling.
In these diseases, Ranivisio can help stabilize and, in many cases, improve your vision.
2. What you need to know before you are given Ranivisio
Ranivisio must not be given to you
- If you are allergic to ranibizumab or any of the other ingredients of this medicine (listed in section 6).
- If you have an infection in the eye or around the eye.
- If you have pain or redness (severe intraocular inflammation) in the eye.
Warnings and precautions
Talk to your doctor before you are given Ranivisio.
- Ranivisio is given by injection into the eye. Occasionally, after treatment with Ranivisio, an infection in the inner eye, pain or redness (inflammation), or a tear or detachment of one of the layers at the back of the eye (retinal detachment or tear and retinal pigment epithelial tear) may occur, or the lens may become cloudy (cataract). It is important to identify and treat such an infection or retinal detachment as soon as possible. Tell your doctor immediately if you notice signs such as eye pain or increased discomfort in the eye, if the redness in the eye gets worse, blurred vision or decreased vision, an increase in the number of small spots in your vision, or increased sensitivity to light.
- In some patients, after the injection, the pressure in the eye may increase for a short time. You may not be aware of this, so your doctor may check your eye pressure after each injection.
- Tell your doctor if you have had eye diseases or have received any treatment in the eyes before, or if you have had a stroke or have had symptoms of a stroke (weakness or paralysis of a limb or face, difficulty speaking or understanding). This information will be taken into account to assess whether Ranivisio is the right treatment for you.
For more detailed information on the side effects that may occur during treatment with Ranivisio, see section 4 ("Possible side effects").
Children and adolescents (under 18 years)
Ranivisio is not recommended for use in children and adolescents, as it has not been established in these age groups.
Other medicines and Ranivisio
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
- Women who may become pregnant should use an effective method of contraception during treatment and for at least three months after the last injection of Ranivisio.
- There is no experience with the use of Ranivisio in pregnant women. Ranivisio should not be used during pregnancy unless the potential benefit outweighs the potential risk to the fetus. If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before treatment with Ranivisio.
- Small amounts of Ranivisio may pass into breast milk, so the use of Ranivisio is not recommended during breastfeeding. Consult your doctor or pharmacist before treatment with Ranivisio.
Driving and using machines
After treatment with Ranivisio, you may experience temporary blurred vision. If this happens, do not drive or use machines until this symptom goes away.
Ranivisio contains polysorbate
This medicine contains 0.005 mg of polysorbate 20 in each administered dose of 0.05 ml, equivalent to 0.10 mg/ml. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergies.
3. How Ranivisio is given
Ranivisio is given by an ophthalmologist as a single injection into the eye under local anesthesia. The usual dose of an injection is 0.05 ml (which contains 0.5 mg of active substance). The pre-filled syringe contains more than the recommended dose of 0.5 mg. The extra volume will not be administered. The excess volume must be expelled before injection. If the entire volume of the pre-filled syringe is injected, it may lead to an overdose.
The interval between two doses given in the same eye must be at least four weeks. All injections will be given by an ophthalmologist.
To prevent infection, before the injection, your doctor will carefully clean your eye. Your doctor will also give you a local anesthetic to reduce or prevent any pain you may feel with the injection.
Treatment is started with an injection of Ranivisio every month. Your doctor will check the disease in your eye and, depending on how you respond to treatment, will decide if you need further treatment and when you need to be treated.
At the end of the leaflet, in the section "How to prepare and administer Ranivisio," detailed instructions for use are provided.
Elderly patients (65 years and older)
Ranivisio can be used in people 65 years of age or older, and no dose adjustment is necessary.
Before stopping treatment with Ranivisio
If you are considering stopping treatment with Ranivisio, go to your next appointment and discuss it with your doctor beforehand. Your doctor will advise you and decide how long you should be treated with Ranivisio.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects associated with the administration of Ranivisio are either due to the medicine itself or the injection procedure, and most affect the eye.
The following are described the most serious side effects:
Common serious side effects(may affect up to 1 in 10 patients)
Detachment or tear of a layer in the inner eye (retinal detachment or tear), resulting in flashes of light with floating particles that progress to transient vision loss or clouding of the lens (cataract).
Uncommon serious side effects(may affect up to 1 in 100 patients) Blindness, infection of the eyeball (endophthalmitis) with inflammation of the inner eye.
The symptoms you may experience are pain or increased discomfort in the eye, worsening redness in the eye, blurred vision or decreased vision, an increase in the number of small spots in your vision, or increased sensitivity to light. Tell your doctor immediately if you experience any of these side effects.
The following are described the most frequently reported side effects:
Very common side effects(may affect more than 1 in 10 patients)
Ocular side effects include: Eye inflammation, bleeding in the back of the eye (retinal hemorrhage), visual disturbances, eye pain, small particles or spots in the vision (floaters), blood in the eye, eye irritation, feeling of having something in the eye, increased tear production, inflammation or infection of the eyelid margin, dry eye, redness or itching of the eye, and increased pressure in the eye.
Non-ocular side effects include: Sore throat, nasal congestion, runny nose, headache, and joint pain.
The following are described other side effects that may occur after treatment with Ranivisio:
Common side effects
Ocular side effects include: Decreased sharpness of vision, swelling of a section of the eye (uvea, cornea), inflammation of the cornea (front part of the eye), small marks on the surface of the eye, blurred vision, bleeding at the injection site, bleeding in the eye, eye discharge with itching, redness, and swelling (conjunctivitis), sensitivity to light, eye discomfort, eyelid swelling, eyelid pain.
Non-ocular side effects include: Urinary tract infection, low red blood cell count (with symptoms such as fatigue, difficulty breathing, dizziness, paleness), anxiety, cough, nausea, allergic reactions such as rash, hives, itching, and redness of the skin.
Uncommon side effects
Ocular side effects include: Inflammation and bleeding in the front part of the eye, accumulation of pus in the eye, changes in the central part of the eye surface, pain or irritation at the injection site, abnormal sensation in the eye, eyelid irritation.
Reporting of side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Ranivisio
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after CAD and on the label of the pre-filled syringe after EXP. The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
- Before use, the sealed tray can be stored at room temperature (25 °C) for a maximum of 24 hours.
- Store the pre-filled syringe in its sealed tray in the carton to protect it from light.
- Do not use any damaged packaging.
6. Container contents and additional information
Ranivisio composition
- The active ingredient is ranibizumab. Each ml contains 10 mg of ranibizumab. A prefilled syringe contains 0.165 ml, equivalent to 1.65 mg of ranibizumab. This provides a usable amount to deliver a single dose of 0.05 ml, which contains 0.5 mg of ranibizumab.
- The other components are α,α-trehalose dihydrate; histidine hydrochloride monohydrate; histidine; polysorbate 20 (E 432); water for injectable preparations.
Product appearance and container contents
Ranivisio is an injectable solution in a prefilled syringe. The prefilled syringe contains 0.165 ml of a sterile, clear, colorless to pale yellow, and aqueous solution. The prefilled syringe contains more than the recommended dose of 0.5 mg. The extractable volume will not be administered in its entirety. The excess volume must be expelled before injection. If the entire volume of the prefilled syringe is injected, it may result in an overdose.
Available pack sizes:
The pack size is a prefilled syringe, packaged in a sealed container tray. The prefilled syringe is for single use.
Marketing authorization holder and manufacturer
Midas Pharma GmbH
Rheinstraße 49
D-55218 Ingelheim
Germany
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A. /AG Tel/Tél: +32 3 820 73 73 | Lietuva UAB Teva Baltics Tel: +370 5 266 02 03 |
| Luxembourg/Luxemburg Teva Pharma Belgium N.V./S.A./AG Tél/Tel: +32 38207373 |
Ceská republika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251 007 111 | Magyarország Teva Gyógyszergyár Zrt. Tel: (+36) 1 288 6400 |
Danmark Teva Denmark A/S Tlf.: +45 44 98 55 11 | Malta Teva Pharmaceuticals Ireland L-Irlanda Tel: +353 (0)1912 7700 |
Deutschland ratiopharm GmbH Tel: +49 731 402 02 | Nederland Teva Nederland B.V. Tel: +31 (0) 800 0228400 |
Eesti UAB Teva Baltics Eesti filiaal Tel.: +372 661 0801 | Norge Teva Norway AS Tlf: +47 66 77 55 90 |
Ελλάδα Specifar ΑΒΕΕ Τηλ: +30 211 880 5000 | Österreich ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
España Teva Pharma, S.L.U. Tel.: + 34 91 387 32 80 | Polska Zaklady Farmaceutyczne Polpharma S.A. Tel. + 48 22 364 61 01 |
France Teva Santé Tél: +33 1 55 91 78 00 | Portugal Teva Pharma - Produtos Farmacêuticos, Lda Tel: +351 21 476 75 50 |
Hrvatska Pliva Hrvatska d.o.o Tel: + 385 1 37 20 000 | România Teva Pharmaceuticals S.R.L Tel: +40 21 230 65 24 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 (0) 207 540 7117 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 1 58 90 390 |
Ísland Teva Pharma Iceland ehf. Sími: + 354 550 3300 | Slovenská republika TEVA Pharmaceuticals Slovakia s.r.o Tel: +421 25726 7911 |
Italia Teva Italia S.r.l Tel:. +39 0289 17981 | Suomi/Finland Ratiopharm Oy Puh/Tel: +358 20 180 5900 |
Κύπρος Specifar ABEE Ελλάδα Τηλ: +30 211880 5000 | Sverige Teva Sweden AB Tel: +46 42 12 11 00 |
Latvija UAB Teva Baltics filiale Latvija Tel: +371 67 323 666 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu
THIS INFORMATION IS INTENDED ONLY FOR HEALTHCARE PROFESSIONALS:
See also section 3 "How to administer Ranivisio".
How to prepare and administer Ranivisio |
Prefilled syringe for single use. For intravitreal use only.
Ranivisio should be administered by an ophthalmologist experienced in the administration of intravitreal injections.
In exudative age-related macular degeneration (AMD), choroidal neovascularization (CNV), retinal pigment epithelial detachment (RPED), and visual impairment due to diabetic macular edema (DME) or edema secondary to branch retinal vein occlusion (BRVO), the recommended dose of Ranivisio is 0.5 mg administered as a single intravitreal injection. This corresponds to an injection volume of 0.05 ml. The interval between two doses injected into the same eye should be at least four weeks.
Treatment is initiated with one injection per month until maximum visual acuity is achieved and/or there are no signs of disease activity, i.e., no change in visual acuity or other signs and symptoms of the disease under continued treatment. In patients with exudative AMD, DME, RPED, and BRVO, three or more consecutive monthly injections may initially be necessary.
From that point on, monitoring and treatment intervals should be determined based on medical judgment and the activity of the disease, assessed by visual acuity and/or anatomical parameters.
Treatment with Ranivisio should be discontinued if, in the physician's judgment, the patient is not benefiting from continued treatment, as evidenced by visual and anatomical parameters.
Monitoring to determine disease activity may include clinical examination, functional testing, or imaging techniques (e.g., optical coherence tomography or fluorescein angiography).
If patients are being treated according to a treat-and-extend regimen, once maximum visual acuity has been achieved and/or there are no signs of disease activity, treatment intervals may be gradually extended until signs of disease activity or visual impairment recur. For exudative AMD, the treatment interval should not be extended by more than two weeks at a time, and for DME, it may be extended up to one month at a time. For RPED and BRVO, treatment intervals may also be gradually extended; however, available data are insufficient to determine the duration of these intervals. If disease activity recurs, the treatment interval should be shortened accordingly.
Treatment of visual impairment due to CNV should be determined on an individual basis for each patient based on disease activity. Some patients may require only one injection during the first 12 months; others may require more frequent treatment, including monthly injections. In the case of CNV secondary to pathologic myopia (PM), many patients may require only one or two injections during the first year.
Ranivisio and laser photocoagulation in DME and edema secondary to branch retinal vein occlusion (BRVO)
There is some experience with ranibizumab administered concomitantly with laser photocoagulation. When administered on the same day, Ranivisio should be administered at least 30 minutes after laser photocoagulation. Ranivisio may be administered in patients who have previously received laser photocoagulation.
Ranivisio and photodynamic therapy with verteporfin in CNV secondary to pathologic myopia (PM)
There is no experience with the concomitant administration of ranibizumab and verteporfin.
Before administering Ranivisio, the absence of particles and discoloration should be checked visually.
The injection procedure should be carried out under aseptic conditions, including surgical hand washing, use of sterile gloves, a sterile field, a sterile blepharostat (or equivalent), and the availability of a sterile paracentesis (if necessary). Before performing the intravitreal injection procedure, the patient's medical history should be thoroughly evaluated for hypersensitivity reactions. Before injection, adequate anesthesia and a broad-spectrum topical microbicidal agent should be administered to disinfect the periocular skin, eyelid, and ocular surface, according to local practice.
The prefilled syringe is for single use. The prefilled syringe is sterile. Do not use the product if the container is damaged. The sterility of the prefilled syringe can only be guaranteed if the tray is kept sealed. Do not use the prefilled syringe if the solution has changed color, is cloudy, or contains particles.
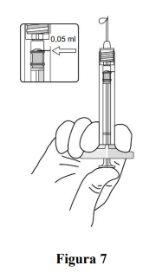
The prefilled syringe contains more than the recommended dose of 0.5 mg. The extractable volume of the prefilled syringe (0.1 ml) will not be administered in its entirety. The excess volume must be expelled before injection. If the entire volume of the prefilled syringe is injected, it may result in an overdose. To expel air bubbles and excess medication, slowly press the plunger until the lower edge of the rubber stopper cap is aligned with the black dosing line on the syringe (equivalent to 0.05 ml, i.e., 0.5 mg of ranibizumab).
For intravitreal injection, a sterile 30G x ½″ injection needle should be used.
This medical device is not included in the packaging.
For the preparation of Ranivisio for intravitreal administration, follow the instructions for use:
Introduction | Read all instructions carefully before using the prefilled syringe. The prefilled syringe is for single use. The prefilled syringe is sterile. Do not use the product if the container is damaged. Opening the sealed tray and the following steps should be performed under aseptic conditions. Note: The dose to be administered should be adjusted to 0.05 ml. | |
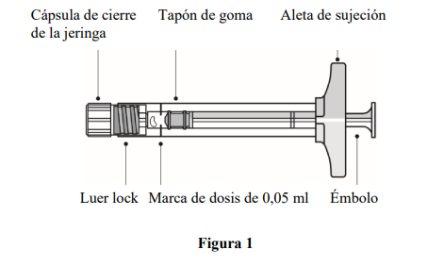
Description of the prefilled syringe |
| |
Prepare |
| |
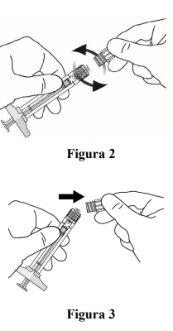
Check the syringe |
| |
Remove the syringe cap |
Discard the syringe cap (see Figure 3). |
|
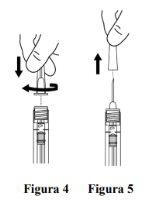
Attach the needle |
Remove the needle cap carefully by pulling it straight off (see Figure 5). Note: Do not wipe the needle at any time. |
|
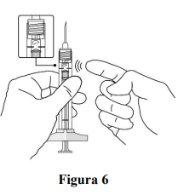
Remove air bubbles |
If there are any air bubbles, gently tap the syringe with your finger until the bubbles rise to the top (see Figure 6). |
|
Adjust the dose |
Note: The plunger is not attached to the rubber stopper – this is to prevent air from entering the syringe. |
|
Inject | The injection procedure should be carried out under aseptic conditions.
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RANIVISIO 10 mg/ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Samsung Bioepis Nl B.V.Prescription requiredDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: INJECTABLE, 10 mg/mLActive substance: ranibizumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for RANIVISIO 10 mg/ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Discuss questions about RANIVISIO 10 mg/ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions