
QUINUX 10 mg/0.4 ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE

How to use QUINUX 10 mg/0.4 ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
QUINUX 10 mg/0.4 ml solution for injection in pre-filled syringe
Methotrexate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.<
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
The frequency and severity of adverse effects will depend on the dose and frequency of administration. It is essential that your doctor performs periodic checks, as severe adverse effects can occur even with the lowest doses. Your doctor will perform tests to control abnormalities that occur in the blood, such as low levels of leukocytes (white blood cells), platelets, lymphoma, and changes in the kidneys and liver.
The most relevant adverse effects related to the administration of Quinux occur in the blood production system and the digestive tract.
Inform your doctor immediately if you experience any of the following symptoms, as they may indicate a severe adverse effect with potential vital danger that may require urgent specific treatment:
- Severe pulmonary disorders (symptoms may be general discomfort, irritating and dry cough, difficulty breathing, feeling of lack of air at rest, chest pain, or fever).
- Blood when coughing or spitting.
- Severe blistering or peeling of the skin.
- Unusual bleeding (including vomiting blood) or bruising.
- Severe diarrhea.
- Ulcers in the mouth.
- Black stools.
- Blood in the urine or stools.
- Small red spots on the skin.
- Fever.
- Yellowing of the skin (jaundice).
- Pain or difficulty urinating.
- Thirst and/or frequent need to urinate.
- Seizures (convulsions).
- Loss of consciousness.
- Blurred vision or decreased vision.
The following adverse effects may occur:
Very common(may affect more than 1 in 10 people):
- Mouth inflammation, indigestion, nausea (vomiting), loss of appetite.
- Increased liver enzymes.
Common(may affect up to 1 in 10 people):
- Mouth ulcers, diarrhea.
- Rash, skin redness, itching.
- Headache, fatigue, drowsiness.
- Decreased formation of red blood cells with decreased number of white blood cells, red blood cells, or platelets (leukopenia, anemia, thrombocytopenia).
Uncommon(may affect up to 1 in 100 people):
- Throat inflammation, intestinal inflammation, vomiting.
- Reactions similar to sunburn due to increased skin sensitivity to sunlight, hair loss, increased number of rheumatic nodules, shingles, blood vessel inflammation, herpes-like rash, hives.
- Onset of diabetes mellitus.
- Dizziness, confusion, depression.
- Inflammation and ulceration of the urinary bladder or vagina, decreased renal function, urinary disorders.
- Joint pain, muscle pain, osteoporosis (reduction of bone mass).
- Decrease in serum albumin.
Rare(may affect up to 1 in 1,000 people):
- Increased skin pigmentation, acne, bruising due to bleeding from blood vessels.
- Allergic reactions, anaphylactic shock, allergic inflammation of blood vessels, fever, red eyes, infection, blood toxicity, altered wound healing, decreased number of antibodies in the blood.
- Visual disturbances.
- Inflammation of the sac around the heart, fluid accumulation in the sac around the heart.
- Low blood pressure.
- Pulmonary fibrosis, pneumonia caused by a specific microorganism (Pneumocystis carinii pneumonia), respiratory difficulty, and bronchial asthma, fluid accumulation in the sac around the lung.
- Electrolyte disturbances.
Very rare(may affect up to 1 in 10,000 people):
- Severe bleeding, toxic megacolon (toxic and acute dilation of the intestine).
- Scalded skin syndrome, increased nail pigmentation, inflammation of the cuticles, furunculosis (deep infection of hair follicles), visible enlargement of small blood vessels.
- Local injury at the injection site (formation of sterile abscesses, changes in fatty tissue) after injection into a muscle or under the skin.
- Visual disturbance, pain, loss of strength or sensation of numbness or tingling/sensitivity to stimuli less than normal, taste disturbances (metallic taste), seizures, paralysis, severe headache with fever.
- Retinopathy (non-inflammatory eye disorder).
- Sudden drop in the number of white blood cells, severe bone marrow depression.
- Lymphoproliferative disorders (excessive increase in white blood cells).
- Loss of sexual appetite, impotence, increased male breast size (gynecomastia), altered sperm formation, menstrual disorders, vaginal discharge.
- Increased size of lymph nodes (lymphoma).
Frequency not known(cannot be estimated from available data):
- Leukoencephalopathy (a disease of the white matter of the brain).
- Pulmonary hemorrhage.
- Jawbone injury (secondary to excessive increase in white blood cells).
- Redness and peeling of the skin.
- Destruction of tissue at the injection site.
- Swelling.
When methotrexate is administered intramuscularly, local adverse reactions (burning sensation) or injuries (formation of sterile abscesses, destruction of fatty tissue) may frequently occur at the injection site. Subcutaneous administration of methotrexate is well tolerated locally. Only mild local skin reactions were observed, which decreased during treatment.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines. https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Quinux
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Keep the pre-filled syringes in the outer packaging to protect them from light. Do not refrigerate or freeze.
Do not use this medicine after the expiration date stated on the packaging after CAD. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE collection point in your pharmacy or any other medicine waste collection system. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Quinux
- The active ingredient is methotrexate. 1 ml of solution contains 25 mg of methotrexate (equivalent to 27.42 mg of methotrexate disodium).
1 pre-filled syringe of 0.4 ml of solution contains 10 mg of methotrexate.
- The other components are sodium chloride, sodium hydroxide, water for injectable preparations.
Appearance of the Product and Package Contents
The pre-filled syringes of Quinux contain a yellowish and transparent solution.
Each package contains 1 or 4 pre-filled syringe(s) with 0.4 ml of solution, with subcutaneous injection needles attached and alcohol-impregnated cotton balls.
Each package contains 1 or 4 pre-filled syringe(s) with 0.4 ml of solution, with subcutaneous injection needles with a safety system attached and alcohol-impregnated cotton balls.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Especialidades Farmacéuticas Centrum, S.A.
C/ Sagitario, 14
03006, Alicante
Spain
Asacpharma Group
Tel.: 965288160; Fax.: 965286434
Manufacturer
Especialidades Farmacéuticas Centrum, S.A.
C/ Sagitario, 14
03006, Alicante
Spain
Date of the Last Revision of this Leaflet: October 2024.
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es)
Instructions for Use
Read the instructions carefully before starting to administer the injection, and always use the application technique advised by your doctor, nurse, or pharmacist.
If you have any problems or questions, contact your doctor, nurse, or pharmacist.
Preparation
Select a clean, flat, and well-lit work surface.
Gather all the necessary elements before starting:
- 1 pre-filled syringe of Quinux
- 1 alcohol-impregnated cotton ball (supplied in the packaging)
Wash your hands carefully. Before using it, inspect the Quinux syringe for visible defects (or cracks).
Injection Site
The best places for injection are:
- the upper part of the thigh,
- the abdomen, except the area around the navel.
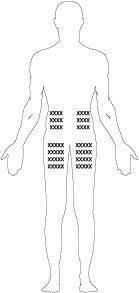
- If someone is helping you with the injection, they can also administer the injection in the back of the arm, just below the shoulder.
- Change the injection site for each injection. This can reduce the risk of developing irritations at the injection site.
- Never administer the injection in painful, bruised, red, hardened, or scarred skin. If you have psoriasis, do not attempt to inject directly into lesions or elevated, thickened, red, or scaly skin patches.
Injection of the Solution
- Open the box containing the pre-filled syringe of methotrexate and read the leaflet carefully. Remove the pre-filled syringe from the packaging at room temperature.
- Disinfection: select an injection site and disinfect it with the alcohol-impregnated cotton ball. Wait 60 seconds for the disinfectant to dry.

- Carefully remove the protective plastic cap, gently twisting it outward. Important: do not touch the needle of the pre-filled syringe.
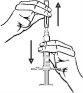
- Insertion of the cannula: with two fingers, form a fold in the skin and quickly insert the needle at a 90-degree angle.
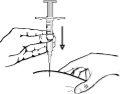
- Injection: insert the needle fully into the skin fold. Slowly push the plunger and inject the liquid under the skin. Hold the skin firmly until the injection is complete. Carefully remove the needle in a straight line.
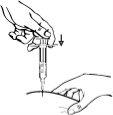
Methotrexate should not come into contact with the skin surface or mucous membranes. In case of contamination, the affected area should be rinsed immediately with plenty of water.
If you or someone else in your environment is injured with the needle, consult your doctor immediately and do not use this pre-filled syringe.
Disposal and Other Handling
Handling and disposal of the medicine and the pre-filled syringe will be carried out in accordance with local regulations for cytotoxic agents. Pregnant healthcare personnel should not handle or administer Quinux.
- Country of registration
- Average pharmacy price13.49 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to QUINUX 10 mg/0.4 ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Online doctors for QUINUX 10 mg/0.4 ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE
Discuss questions about QUINUX 10 mg/0.4 ml INJECTABLE SOLUTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






