
BERTANEL 20 mg/1 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use BERTANEL 20 mg/1 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Bertanel and what is it used for
- What you need to know before you use Bertanel
- How to use Bertanel
- Bertanel should only be prescribed by doctors familiar with the different characteristics of the medication and its mode of action.
- Possible side effects
- Conservation of Bertanel
- Packaging contents and additional information
- BG
Introduction
Package Leaflet: Information for the Patient
Bertanel20 mg/1 ml Solution for Injection in Pre-filled Syringe
methotrexate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Bertanel and what is it used for
- What you need to know before you use Bertanel
- How to use Bertanel
- Possible side effects
- Storage of Bertanel
- Contents of the pack and other information
1. What is Bertanel and what is it used for
Bertanel is a medicine that has the following properties:
- interferes with the growth of certain body cells that multiply rapidly (antitumour agent)
- reduces unwanted reactions of the body's own defence mechanism (immunosuppressant) and
- has anti-inflammatory effects
Bertanel is used in patients with:
- active rheumatoid arthritis (RA) in adult patients.
- polyarticular forms (when five or more joints are involved) of active and severe juvenile idiopathic arthritis (JIA) when the response to non-steroidal anti-inflammatory drugs (NSAIDs) has been inadequate.
severe disabling psoriasis that is not adequately responsive to other forms of therapy such as phototherapy, PUVA, and retinoids, and severe psoriasis that affects the joints (psoriatic arthritis) in adult patients.
2. What you need to know before you use Bertanel
Please ask your doctor or pharmacist before using Bertanel if you have any questions. |
Do not use Bertanel:
- if you are allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6)
- if you have severe kidney disease (your doctor decides the severity of the disease)
- if you have severe liver disease (your doctor decides the severity of your disease)
- if you have blood cell disorders
- if you have high alcohol consumption
- if you have an altered immune system
- if you have an existing or severe infection, e.g. tuberculosis and HIV
- if you have active gastrointestinal ulcers (including oral ulcers)
- if you are pregnant or breastfeeding (see section "Pregnancy, breastfeeding and fertility")
- if you are receiving live virus vaccines at the same time.
Warnings and precautions
Consult your doctor before starting Bertanel if you:
- have insulin-treated diabetes mellitus
- have chronic inactive infections (e.g. tuberculosis, hepatitis B or C, herpes (herpes zoster))
- have or have had liver or kidney disease
- have lung function problems
- have abnormal fluid accumulation in the abdomen or in the space between the lungs and the chest wall (ascites, pleural effusion)
- are dehydrated or have a condition that may cause dehydration (vomiting, diarrhoea, stomatitis).
Acute pulmonary haemorrhage has been reported with methotrexate in patients with underlying rheumatologic disease. If you cough up blood or spit up blood, you should contact your doctor immediately.
Treatment is administered once a week.
Incorrect administration of methotrexate can cause serious side effects, including potentially life-threatening ones.
Read section 3 of this leaflet carefully.
Methotrexate can make your skin more sensitive to sunlight. Avoid intense sunlight and do not use sunbeds or UV lamps without medical advice. To protect your skin from intense sunlight, wear suitable clothing or use a sunscreen with a high protection factor.
If you have had skin problems after radiotherapy (radiation-induced dermatitis) and sunburn, these conditions may recur during treatment with methotrexate (memory reaction).
If you, your partner, or your caregiver notice the onset or worsening of neurological symptoms, such as general muscle weakness, vision changes, changes in thinking, memory, and orientation that cause confusion, and personality changes, contact your doctor immediately because these may be symptoms of a rare and serious brain infection called progressive multifocal leukoencephalopathy (PML).
Children and adolescents
Administration instructions depend on the patient's body weight. The use in children under 3 years of age is not recommended due to insufficient experience in this age group.
Children treated with Bertanel should be closely monitored by a specialist doctor to identify possible side effects as soon as possible.
Elderly patients
Elderly patients treated with methotrexate should be under close medical supervision by a specialist to detect possible side effects as soon as possible.
The deterioration of liver and kidney function associated with age, as well as low folate reserves in old age, require the administration of a relatively low dose of methotrexate.
Skin lesions caused by psoriasis may worsen during treatment with Bertanel if exposed to UV radiation at the same time.
Recommended investigations and precautions:
Even when Bertanel is administered at low doses, serious side effects can occur. To detect them as soon as possible, your doctor should perform follow-up examinations and laboratory tests.
Before starting treatment:
Before starting treatment, you will have a blood test to check if you have enough blood cells. Your liver function and hepatitis will also be checked in your blood. The serum albumin (a blood protein), hepatitis status (liver infection), and kidney function will also be checked. Your doctor may decide to perform other liver tests, some of which may require images of your liver or a small tissue sample from your liver to examine it more closely. Your doctor may also check if you have tuberculosis and may perform a chest X-ray or lung function test.
During treatment:
Your doctor may perform the following examinations:
- examination of the oral cavity and pharynx to detect changes in the mucous membrane, such as inflammation or ulcers
- blood test/blood count, with blood cell count and measurement of serum methotrexate levels
- blood test to monitor liver function
- imaging tests to monitor liver function
- taking a small tissue sample from the liver to examine it more closely
- blood test to monitor kidney function
- monitoring of the respiratory tract and, if necessary, lung function test
It is very important that you undergo these scheduled tests.
If the results of any of these tests are remarkable, your doctor will adjust the treatment accordingly.
Special precautions for treatment with Bertanel
Methotrexate temporarily affects sperm and egg production, which is reversible in most cases. Methotrexate can cause abortions and severe birth defects. If you are a woman, you should avoid becoming pregnant while using methotrexate and for at least six months after stopping treatment. If you are a man, you should avoid fathering a child while being treated with methotrexate and for at least 3 months after stopping treatment. See also section "Pregnancy, breastfeeding and fertility".
Using Bertanel with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. Remember to inform your doctor about your treatment with Bertanel if you are prescribed another medicine while still being treated.
It is especially important that you inform your doctor if you are using:
- other treatments for rheumatoid arthritis or psoriasis, such as leflunomide, sulfasalazine (also used for ulcerative colitis), aspirin, phenylbutazone, or aminopyrine
- alcohol (should be avoided)
- live virus vaccines
- azathioprine (used to prevent organ rejection after a transplant)
- retinoids (used to treat psoriasis and other skin diseases)
- anticonvulsants (prevent epileptic seizures)
- cancer treatments
- barbiturates (help with sleep)
- tranquillisers
- oral contraceptives
- probenecid (for gout)
- antibiotics
- Penicillins may reduce the excretion of methotrexate, potentially increasing the risk of side effects
- metamizole (synonyms novaminsulfon and dipyrone) (medicine for severe pain and/or fever)
- pyrimethamine (used to prevent and treat malaria)
- vitamin preparations containing folic acid
- proton pump inhibitors (used to treat stomach acid or ulcers)
- theophylline (used to treat asthma)
Using Bertanel with food, drinks, and alcohol
During treatment with Bertanel, you should not consume alcohol and should avoid excessive consumption of coffee, caffeine-containing soft drinks, and black tea.
You should ensure a high intake of fluids during treatment with Bertanel, as dehydration (reduction of body water) can increase the toxicity of Bertanel.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
Do not use Bertanel during pregnancy or if you are trying to become pregnant. Methotrexate can cause birth defects, harm the fetus, or cause miscarriages. It is associated with malformations of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that methotrexate is not administered to pregnant women or women who plan to become pregnant. In women of childbearing age, any possibility of pregnancy should be excluded with appropriate measures, for example, a pregnancy test before starting treatment.
You should avoid becoming pregnant while taking methotrexate and for at least 6 months after stopping treatment, using reliable contraceptive methods during this time (see also section "Warnings and precautions").
If you become pregnant during treatment or think you may be pregnant, consult your doctor as soon as possible. You should be informed about the risk of harmful effects on the child during treatment.
If you wish to become pregnant, consult your doctor, who may refer you to a specialist for advice before the planned start of treatment.
Breastfeeding
You should not breastfeed while using Bertanel, as methotrexate passes into breast milk. If your doctor considers that treatment with methotrexate is absolutely necessary, breastfeeding should be interrupted.
Male fertility
Available data do not indicate an increased risk of malformations or miscarriages if the father takes a dose of methotrexate less than 30 mg/week. However, this risk cannot be completely ruled out. Methotrexate can be genotoxic, which means it can cause genetic mutations. Methotrexate can affect sperm production and cause birth defects. Therefore, you should avoid fathering a child or donating sperm while taking methotrexate and for at least 3 months after stopping treatment.
Driving and using machines
During treatment with Bertanel, side effects that affect the central nervous system, such as fatigue and dizziness, may occur. In some cases, the ability to drive vehicles and/or use machines may be impaired. If you feel tired or dizzy, do not drive or operate tools or machines.
Bertanel contains sodium chloride and sodium hydroxide
This medicine contains less than 1 mmol of sodium (23 mg) per weekly dose; this is essentially "sodium-free".
3. How to use Bertanel
Important dosage warning for Bertanel (methotrexate):
Use Bertanel only once a weekfor the treatment of rheumatoid arthritis, polyarticular forms of juvenile idiopathic arthritis, or psoriasis. Excessive use of Bertanel (methotrexate) can be fatal. Read section 3 of this leaflet carefully. If you have any doubts, consult your doctor or pharmacist before taking this medicine.
Bertanel should only be prescribed by doctors familiar with the different characteristics of the medication and its mode of action.
Follow the administration instructions of this medication exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Bertanel is administered onlyonce a week. Along with your doctor, you will decide on a suitable day each week to receive your injection.
Incorrect administration of Bertanel can produce serious side effects, including potentially life-threatening ones.
The normal dose is:
Dosage in patients with rheumatoid arthritis
The recommended initial dose is 7.5 mg of methotrexate once a week. Bertanel is administered in a single application in the form of an injection under the skin, in a muscle, or in a vein (see section "Method and duration of administration").
If this is not sufficient and if you tolerate the medication well, the doses of Bertanel may be increased. The average weekly dose is 15-20 mg. Generally, a weekly dose of 25 mg of Bertanel should not be exceeded. Once the desired therapeutic result is achieved, if possible, the dose should be gradually reduced to the lowest effective maintenance dose.
Dosage in children and adolescents under 16 years of age with polyarticular forms of juvenile idiopathic arthritis
The recommended dose is 10-15 mg/m2 of body surface area per week. In cases of inadequate response, the weekly dose may be increased up to 20 mg/m2 of body surface area per week. However, more frequent monitoring should be performed. Since there is very little data on intravenous administration (in a vein) in children and adolescents, it should only be administered via subcutaneous (under the skin) or intramuscular (in the muscle) injection.
Use in children under 3 years of age is not recommended, as experience in this age group is insufficient.
Adults with severe forms of psoriasis vulgaris or psoriatic arthritis
A test dose of 5-10 mg is recommended to evaluate potential adverse effects.
This dose can be administered subcutaneously (under the skin), intramuscularly (in the muscle), or intravenously (in a vein).
If, one week later, no changes in blood count have been observed, treatment will continue with a dose of approximately 7.5 mg. The dose can be progressively increased (in stages of 5-7.5 mg per week and monitoring the blood count) until the desired therapeutic results are achieved. Generally, a weekly dose of 20 mg can be associated with significant increases in toxicity. A weekly dose of 30 mg should not be exceeded.
Once the desired therapeutic result is achieved, the dose should be reduced weekly until the lowest effective maintenance dose is reached for the patient.
Patients with renal insufficiency
Patients with renal insufficiency may require lower doses.
Method and duration of administration
Your doctor will determine the duration of treatment. Bertanel is injected once a week!It is recommended to specify a particular day of the week as the "injection day".
Bertanel is administered in the form of an injection under the skin, in a muscle, or in a vein; in children and adolescents, it should not be administered intravenously.
Treatment with Bertanel for rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis vulgaris, and psoriatic arthritis is long-term treatment.
Rheumatoid arthritis
Improvement of symptoms can usually be expected after 4-8 weeks of treatment. Symptoms may reappear after discontinuation of treatment with Bertanel.
Severe forms of psoriasis vulgaris and psoriatic arthritis (psoriatic arthropathy)
Response to treatment can usually be expected after 2-6 weeks.
Depending on the clinical picture and changes in laboratory parameters, treatment will be continued or discontinued.
At the start of treatment, Bertanel may be injected by medical personnel. However, it is possible that your doctor may decide that you can learn to inject Bertanel yourself. You will receive adequate training for this. Under no circumstances should you attempt to inject yourself unless you have been taught to do so.
If you use more Bertanel than you should
Follow the dosage recommendations given by your doctor. Do not change the dose on your own.
If you suspect that you (or someone else) have administered too much Bertanel, please contact your doctor or go immediately to the nearest hospital or consult the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount used. He/She will decide on the necessary treatment measures, depending on the severity of the poisoning.
An overdose of methotrexate can produce severe toxic reactions. Symptoms of overdose may include bleeding or bruising, unusual weakness, sores in the mouth, nausea, vomiting, black or bloody stools, coughing up blood or vomit resembling coffee grounds, and decreased urine production. See section 4.
Bring the medication packaging with you if you go to the doctor or hospital.
The antidote in case of overdose is calcium folinate.
If you forget to use Bertanel
Do not use a double dose to make up for forgotten doses; continue using the prescribed dose. If you have doubts, consult your doctor.
If you interrupt treatment with Bertanel
You should not interrupt treatment with Bertanel unless your doctor has indicated it. If you suspect serious adverse effects, consult your doctor immediately.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can produce side effects, although not all people suffer from them.
Inform your doctor immediately if you experience wheezing, difficulty breathing, swelling of the eyelids, face, or lips, rash, or itching (especially if it affects the whole body) and if you feel like you are going to faint (these could be symptoms of severe allergic reactions or anaphylactic shock).
Severe side effects
If any of the following side effects appear, contact your doctor immediately:
- lung problems (symptoms may be general discomfort, irritating and dry cough, difficulty breathing, feeling of lack of air at rest, chest pain, or fever)
- blood when coughing or spitting
- blistering or severe peeling of the skin (this can also affect the mouth, eyes, and genitals)
- unusual bleeding (including vomiting blood) or bruising
- severe diarrhea
- mouth ulcers
- black or tarry stools
- blood in urine or stools
- small red spots on the skin
- fever
- yellowing of the skin (jaundice)
- pain or difficulty urinating
- swelling of hands, ankles, or feet, or changes in urination frequency or decreased or absent urine (symptoms of kidney failure)
- thirst and/or need to urinate frequently
- seizures (convulsions)
- loss of consciousness
- blurred vision or decreased vision
The following side effects have also been reported:
Very common (may affect more than 1 in 10 people):
- loss of appetite, nausea (feeling dizzy), vomiting, abdominal pain
- inflammation and ulcers in the mouth and throat
- increased liver enzymes.
Common (may affect up to 1 in 10 people):
- reduced formation of blood cells with decreased white blood cells and/or red blood cells and/or platelets (leukopenia, anemia, thrombocytopenia)
- headache
- fatigue, drowsiness
- tingling, numbness, or burning sensation of the skin, hives, redness of the skin, itching
- inflammation of the lungs (pneumonitis)
- diarrhea.
Uncommon (may affect up to 1 in 100 people):
- shingles (herpes zoster)
- lymphoma (which in some cases spontaneously resolved as soon as treatment with Bertanel was discontinued)
- decrease in the number of blood cells and platelets
- severe allergic reactions
- diabetes
- depression
- weakness of the entire left or right side of the body
- dizziness, confusion
- seizures
- brain damage (leukoencephalopathy/encephalopathy)
- inflammation of blood vessels
- lung damage, pulmonary edema
- ulcers and bleeding in the digestive tract
- inflammation of the pancreas
- liver disorders
- decrease in blood proteins
- hives (alone), sensitivity to light, brown discoloration of the skin
- severe toxic skin reactions including blistering and peeling of the skin (Stevens-Johnson syndrome, Lyell syndrome)
- hair loss
- increase in rheumatoid nodules
- psoriasis with pain
- reactions similar to sunburn due to increased skin sensitivity to sunlight
- muscle or joint pain
- osteoporosis (reduction of bone mass)
- inflammation and ulcers of the bladder (possibly with blood in the urine), pain when urinating
- fetal malformations
- inflammation and ulcers in the vagina
- burning sensation or tissue damage after injection of Bertanel into the muscle.
Rare (may affect up to 1 in 1,000 people):
- sepsis
- very large red blood cells (megaloblastic anemia)
- mood changes
- temporary problems with perception
- weakness of voluntary movement in the whole body
- speech problems
- severe eye problems
- low blood pressure
- blood clots
- sore throat
- interrupted breathing
- inflammation of the digestive tract, bloody stools
- inflammation of the gums
- acute hepatitis (inflammation of the liver)
- changes in nail color, nail loss
- acne, red or purple spots due to broken blood vessels
- bone fractures due to stress
- electrolyte disturbances
- abortion
- defective sperm formation
- menstrual disorders.
Very rare (may affect up to 1 in 10,000 people):
- cold sores (herpes simplex)
- hepatitis
- severe bone marrow failure
- immunodeficiency (hypogammaglobulinemia)
- pain
- muscle weakness
- taste disturbances (metallic taste)
- inflammation of the brain lining that causes paralysis or vomiting
- red eyes
- inflammation of the sac surrounding the heart, fluid in the sac surrounding the heart
- pneumonia, respiratory problems, asthma
- vomiting blood
- liver failure
- infection around the nails, boils, small blood vessels in the skin
- proteins in the urine
- fetal death
- problems with ovule formation (in women) and sperm formation (in men)
- loss of sexual desire
- erection problems
- vaginal discharge
- infertility
- mild local reactions on the skin if Bertanel is administered subcutaneously
- lymphoproliferative disorders (excessive increase in white blood cells)
- numbness or tingling/sensitivity to stimuli less than normal
Frequency not known (cannot be estimated from the available data):
- infections that can be life-threatening in some cases
- inflammation of lymph nodes
- malfunction of the immune system
- fever
- inflammation of small blood vessels caused by an allergic reaction
- inflammation of the abdominal lining
- slow wound healing
- pulmonary hemorrhage
- damage to the jawbone (secondary to an excessive increase in white blood cells)
- destruction of tissue at the injection site
- redness and peeling of the skin
- swelling
When methotrexate is administered intramuscularly, local adverse reactions (burning sensation) or lesions (formation of sterile abscesses, destruction of fatty tissue) can frequently occur at the injection site. Subcutaneous administration of methotrexate has good local tolerance. Only mild local skin reactions have been observed, decreasing during treatment.
Methotrexate can reduce the number of white blood cells, which can decrease resistance to infection. If you suffer from an infection with symptoms such as fever and severe deterioration of your general condition, or fever with symptoms of local infection such as sore throat/pharyngitis/mouth pain or urinary problems, you should see your doctor immediately. A blood test will be performed to check for a possible decrease in white blood cells (agranulocytosis). It is essential to inform your doctor about your medication.
Methotrexate can produce severe side effects (sometimes life-threatening). Therefore, your doctor will perform tests to check for anomalies caused in the blood (e.g., decreased white blood cells, decreased platelets, lymphoma) and changes in the kidneys and liver.
Reporting of side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that does not appear in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Bertanel
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date that appears on the label of the pre-filled syringe and on the packaging after "EXP". The expiration date is the last day of the month indicated.
Store in the original packaging to protect it from light.
Do not store at a temperature above 25°C.
The product must be used immediately after opening the packaging.
Do not use Bertanel if the solution is not transparent and contains particles.
For single use. Discard any unused solution!
Medications should not be thrown away through the sewers or in the trash. Deposit the packaging and medications you no longer need at the SIGRE Point in the pharmacy. If you have doubts, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Packaging contents and additional information
Composition of Bertanel
The active ingredient is methotrexate.
1 ml of injectable solution contains 20 mg of methotrexate (equivalent to 21.94 mg of methotrexate disodium).
1 pre-filled syringe of 1 ml of injectable solution contains 20 mg of methotrexate.
The other components are: sodium chloride, sodium hydroxide for pH adjustment, and water for injectable preparations.
Appearance of the product and packaging contents
Bertanel is an injectable solution that is marketed in pre-filled syringes with a transparent yellowish solution for injection.
Each box contains 1 pre-filled syringe or multipack packaging containing 4, 5, 6, 12, or 30 pre-filled syringes with 1 ml of injectable solution, single-use injection needles with or without a safety cannula, and alcohol-soaked cotton balls.
Only some packaging sizes may be marketed.
Marketing authorization holder
Ebewe Pharma Ges.m.b.H. Nfg.KG
Mondseestrasse 11
4866 Unterach, Austria
Manufacturer
Ebewe Pharma Ges.m.b.H. Nfg.KG
Mondseestrasse 11
4866 Unterach, Austria
Salutas Pharma GmbH
Otto-von-Guericke-Alle 1
D-39179 Barleben
Germany
Fareva Unterach GmbH
Mondseestraße 11
4866 Unterach
Austria
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Local representative
Laboratorios Farmacéuticos ROVI, S.A.
Julián Camarillo, 35
28037 Madrid
Spain
This medication is authorized in the member states of the European Economic Area with the following names:
Member state name | Medication name | Holder |
AT | Ebetrexat 20mg/ml Injektionslösung in einer Fertigspritze | EBEWE Pharma Ges.m.b.H. Nfg. KG |
BE | Ebetrexat 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
BG | Ebetrexat 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
CZ | Methotrexat Ebewe 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
DE | MTX Sandoz 20mg/ml injection solution, in a pre-filled syringe | Sandoz Pharmaceuticals GmbH |
DK | Ebetrex | EBEWE Pharma Ges.m.b.H. Nfg. KG |
ES | Bertanel 20 mg/1 ml injectable solution in a pre-filled syringe. | EBEWE Pharma Ges.m.b.H. Nfg. KG |
EE | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
FI | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
HU | Ebetrexat 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
IT | TREXODEM 20mg/ml injectable solution, pre-filled syringes | Sandoz S.p.A. |
LT | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
LU | Methotrexat Sandoz 20mg/ml, solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
LV | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
NL | Ebetrex 20 mg = ml, solution for injection in a pre-filled syringe 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
NO | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
PL | Ebetrexat | EBEWE Pharma Ges.m.b.H. Nfg. KG |
PT | Methotrexato Sandoz, injectable solution, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
RO | Methrotrexate Ebewe 20 mg/ml injectable solution in a pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
SE | Ebetrex 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
SK | Methotrexat Ebewe 20mg/ml | EBEWE Pharma Ges.m.b.H. Nfg. KG |
SI | Metotreksat “Ebewe” 20 mg/ml solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
UK | Ebetrex 20mg/ml solution for injection, pre-filled syringe | EBEWE Pharma Ges.m.b.H. Nfg. KG |
Date of the last revision of this prospectus:September 2024
The detailed and updated information of this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Bertanel 20 mg/1 ml injectable solution in a pre-filled syringe
Instructions for use, handling, and disposal
The solution must be transparent and particle-free.
Handling and disposal of the medication must be carried out in the same way as with other cytotoxic preparations and in accordance with local regulations. If any female healthcare worker is pregnant, she should not handle and/or administer Bertanel.
For single use. Discard any unused solution.
Disposal of unused medication or waste materials will be carried out in accordance with local regulations.
Incompatibilities
In the absence of compatibility studies, this medication should not be mixed with others.
Special precautions for storage
Store in the original packaging to protect it from light.
Do not store at a temperature above25°C.
Step-by-step instructions for subcutaneous injection:
Step 1:
- Remove the inner container containing the pre-filled syringe, cannula, and needle from the box.
- Open the inner container by pulling the flap at the corner. Remove the pre-filled syringe.
- Turn the gray rubber cap covered with plastic on the syringe, without touching the opening of the pre-filled syringe (see Figure 1).
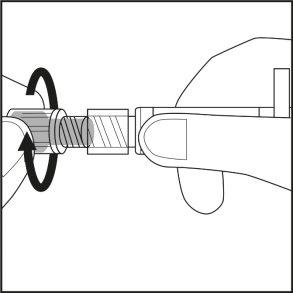
Figure 1.
Step 2:
- Place the syringe back in the inner container. The yellow solution will not spill.
- Check the label on the plastic case containing the needle. The label must be intact (see Figure 2).
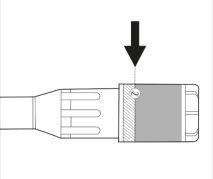
Figure 2.
Step 3:
- Remove the cap from the plastic case of the needle by turning and pulling it. See Figure 3.1
- Carefully turn the needle along with the plastic case onto the syringe until it reaches the end. See Figure 3.2
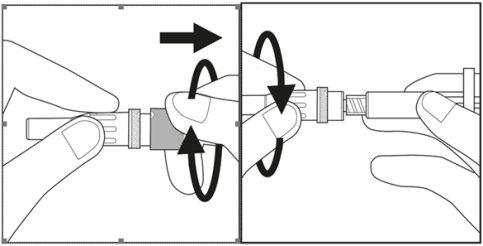
Figure 3.1 Figure 3.2
Step 4:
- Choose the injection site on the abdomen or thigh and clean it with a cotton swab soaked in alcohol. Do not touch this area before injection (see Figures 4.1 and 4.2).
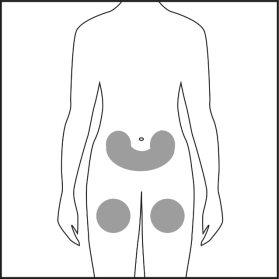
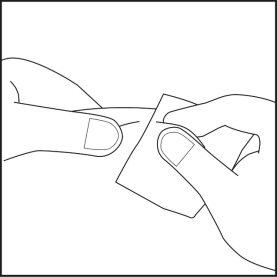
Figure 4.1 Figure 4.2
Step 5:
- Remove the sheath from the cannula and set it aside.
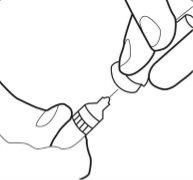
- Do not touch the sterile cannula. If this happens, ask your doctor or pharmacist about the possibility of using another cannula. With two fingers, form a fold in the skin and then insert the needle into it almost vertically.
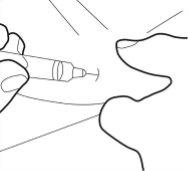
Step 6:
- Insert the cannula completely into the skin fold. Then, slowly push the plunger down and inject all the liquid under the skin.
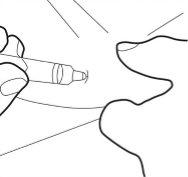
Step 7:
- Remove the cannula carefully and wipe the injection site with a cotton swab soaked in alcohol. Do not rub as it may cause irritation at the injection site.
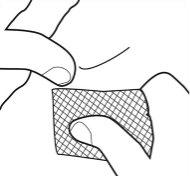
- To avoid injury, discard used syringes in a container.
- Country of registration
- Average pharmacy price26.62 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BERTANEL 20 mg/1 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 25 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Online doctors for BERTANEL 20 mg/1 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about BERTANEL 20 mg/1 ml PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






