
PRISMASOL 4 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION


How to use PRISMASOL 4 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Prismasol 4 mmol/l Potassium solution for haemodialysis and haemofiltration.
Calcium chloride, dihydrate / Magnesium chloride hexahydrate / Glucose monohydrate / 90% lactic acid solution / Sodium chloride / Potassium chloride / Sodium hydrogen carbonate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Prismasol is and what it is used for.
- What you need to know before you use Prismasol.
- How to use Prismasol
- Possible side effects
- Storage of Prismasol
- Contents of the pack and other information
1. What Prismasol is and what it is used for
Prismasol contains the following active substances: calcium chloride, dihydrate; magnesium chloride, hexahydrate; glucose monohydrate, 90% lactic acid solution; sodium chloride; potassium chloride and sodium hydrogen carbonate.
Prismasol is used in the treatment of renal failure as a solution for haemofiltration or haemodiafiltration (as a replacement for the loss of fluid produced by the passage of blood through the filter) and haemodialysis (the blood passes through one side of the dialysis membrane while the dialysis solution passes through the other side of the same membrane).
Prismasol can also be used in case of poisoning with dialysable or filterable substances.
Prismasol 4 mmol/l Potassium is especially indicated in patients with normal potassium levels (normal potassium concentration in blood).
2. What you need to know before you start using Prismasol
Do not use Prismasol 4 mmol/l Potassium in case of:
- allergy to any of the active substances or to any of the other ingredients (listed in section 6),
- high level of potassium in the blood (hyperkalaemia),
- high concentration of bicarbonate in the blood (metabolic alkalosis).
The presence of antigen derived from maize in Prismasol cannot be excluded.
Do not use haemofiltration or dialysis treatment in case of:
- Renal failure with pronounced hypercatabolism (abnormally increased catabolism), if the uraemic symptoms (symptoms caused by a high concentration of urea in the blood) cannot be corrected with haemofiltration.
- Insufficient blood pressure in the vascular access.
- Systemic anticoagulation (reduced blood coagulation) if there is a high risk of bleeding.
Warnings and precautions
Consult your doctor, pharmacist or nurse before you start using Prismasol.
The solution can only be used under the supervision of a competent doctor in the treatment of renal failure using haemofiltration, haemodiafiltration and haemodialysis techniques.
Before and during treatment, the blood condition will be monitored, for example, the acid-base balance and the concentration of electrolytes (salts in the blood) will be checked, as well as all the fluids that are administered (intravenous perfusion) and produced (diuresis), even those not directly related to the treatment.
The glucose concentration in the blood should be closely monitored, especially if you are diabetic.
Other medicines and Prismasol
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The concentration in the blood of some of the other medicines may be reduced during treatment. Your doctor will decide if your medication needs to be changed.
In particular, inform your doctor in the following cases:
- Digitalis medicines (for the treatment of certain heart failures) since the risk of cardiac arrhythmias (irregular or rapid heartbeats) induced by these medicines increases during hypokalaemia (low potassium concentration in the blood).
- Vitamin D and medicines containing calcium, since they may increase the risk of hypercalcaemia (elevated calcium concentration in the blood).
- Any sodium hydrogen carbonate supplement (or other buffered source), since it may increase the risk of metabolic alkalosis (excess of bicarbonate in the blood).
- When citrate is used as an anticoagulant (as a protector in dialysis equipment), it may reduce the calcium levels in plasma.
Pregnancy and Breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
If you are pregnant or breast-feeding, your doctor will decide if you should be given Prismasol.
Driving and using machines
There is no evidence to suggest that Prismasol affects your ability to drive or use machines.
3. How to use Prismasol
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The volume of Prismasol to be administered will depend on your clinical conditions and the desired fluid balance. Therefore, the dose volume will depend on your doctor's judgement.
Route of administration: intravenous and haemodialysis.
If you think you have used more Prismasol than you should:
Your fluid, acid-base and electrolyte balance must be carefully monitored.
In the unlikely event of an overdose, your doctor will take the necessary corrective measures to adjust the dose.
An overdose may cause:
- fluid overload in the blood,
- increased bicarbonate level in the blood (metabolic alkalosis),
- and/or reduced salt levels in the blood (hypophosphataemia, hypokalaemia).
Overdose could lead to serious consequences such as congestive heart failure, electrolyte or acid-base imbalance.
For instructions on use, see the section "This information is intended only for healthcare professionals".
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported:
Unknown: frequency cannot be estimated from the available data
- Changes in salt levels in the blood (electrolyte imbalances such as hypophosphataemia)
- Increased bicarbonate concentration in plasma (metabolic alkalosis) or reduced bicarbonate concentration in plasma (metabolic acidosis)
- Abnormally high or low water volume in the body (hyper- or hypovolaemia)
- Abnormally high glucose concentration in the blood (hyperglycaemia)
- Nausea
- Vomiting
- Muscle cramps
- Hypotension (low blood pressure)
- Hypercalcaemia (elevated calcium concentration in the blood)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse, even if it is possible that they are not listed in this leaflet.
You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Prismasol
Keep this medicine out of the sight and reach of children.
Do not store below +4°C.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
The chemical and physical stability of the reconstituted solution has been demonstrated for 24 hours at a temperature of 22°C. If the solution is not used immediately, the in-use shelf-life and storage conditions before use are the responsibility of the user and should not exceed 24 hours, including the duration of the treatment.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help to protect the environment.
6. Container Contents and Additional Information
PVC FORMAT WITH BREAKABLE VALVE
What Prismasol contains
The active ingredients are:
Before reconstitution:
1000 ml of Electrolyte Solution (Small Compartment A) contains:
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose 22.000 g
(S)-Lactic acid 5.400 g
1000 ml of Buffer Solution (Large Compartment B) contains:
Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
Potassium chloride 0.314 g
After reconstitution:
The solution from Compartment A (250 ml) and Compartment B (4750 ml) is mixed to produce a reconstituted solution (5000 ml) with the following composition:
mmol/l | mEq/l | |
Calcium Ca2+ | 1.75 | 3.50 |
Magnesium Mg2+ | 0.50 | 1.00 |
Sodium Na+ | 140.00 | 140.00 |
Chloride Cl- | 113.50 | 113.50 |
Lactate | 3.00 | 3.00 |
Sodium hydrogen carbonate HCO3- | 32.00 | 32.00 |
Potassium K+ | 4.00 | 4.00 |
Glucose | 6.10 | |
Theoretical osmolality: | 301 mOsm/l |
The other components are:carbon dioxide (E-290), water for injectable preparations.
pH of the reconstituted solution: 7.0-8.5
Appearance of the product and container contents
Prismasol is presented in a bicompartmental bag, containing the small Compartment A with the electrolyte solution and the large Compartment B with the buffer solution. The final reconstituted solution is obtained after breaking the breakable valve and mixing both solutions. The reconstituted solution is transparent and slightly yellow. Each bag (A+B) contains 5000 ml of solution for hemodialysis and hemofiltration. The bag is covered by a transparent overbag.
Each box contains two bags and a leaflet.
Marketing Authorization Holder:
Vantive Belgium SRL
Boulevard d'Angleterre 2
1420 Braine-l'Alleud
Belgium
Manufacturer:
Bieffe Medital S.p.A.
Via Stelvio 94
23035 Sondalo (SO)
Italy
Or
Vantive Manufacturing Limited
Moneen Road,
Castlebar
County Mayo
F23 XR63
Ireland
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany, Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Slovakia, Slovenia, Estonia, Spain, Finland, France, Greece, Netherlands, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Portugal, Romania, Sweden, United Kingdom (Northern Ireland): Prismasol 4
For further information on this medicinal product, please contact the local representative of the Marketing Authorization Holder:
Vantive Health, S.L.
Industrial Estate Sector 14
C/ Pouet de Camilo, 2
46394 Ribarroja del Turia (Valencia)
Spain
Date of last revision of this leaflet: 03/2018
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es
-----------------------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only
Prismasol 4 mmol/l Potassium Solution for Hemodialysis and Hemofiltration
Precautions
The instructions for use and handling of Prismasol must be followed accurately.
The solutions from the two compartments must be mixed before use.
The use of contaminated hemofiltration and hemodialysis solutions can cause sepsis, shock, and conditions that can lead to death.
Prismasol can be warmed to 37°C to improve patient comfort. The warming of the solution before use must be done before reconstitution, using only dry heat. The solutions must not be warmed in water or in the microwave. The solution must be visually inspected before administration to detect the presence of particles and any possible color change, when the solution and container allow it. Do not administer if the solution is not transparent or if the seal is not intact.
Prismasol is a solution that contains potassium. The solution should not be administered to patients with hyperkalemia. Before and during hemofiltration and/or hemodialysis, the blood potassium concentration should be monitored.
If hyperkalemia occurs once treatment has started, the addition of potassium sources that affect concentrations should be assessed. When the solution is used as a replacement solution, the perfusion rate should be decreased and it should be confirmed that the desired potassium concentration has been reached. If hyperkalemia is not resolved, perfusion should be stopped immediately.
If hyperkalemia develops when using the solution as dialysate, it may be necessary to administer a potassium-free dialysate to increase potassium elimination.
The concentration of inorganic phosphates should be measured regularly. In the event that blood inorganic phosphate levels are low, they should be restored. A phosphate concentration of up to 1.2 mmol/l can be added to the solution. If phosphate is added to the bag, sodium phosphate should be used.
Although no cases of severe hypersensitivity reactions to corn with Prismasol have been reported, solutions containing glucose derived from hydrolyzed corn starch should not be used in patients with known allergy to corn or corn-derived products.
If signs or symptoms of suspected hypersensitivity reactions develop, administration should be interrupted immediately. Appropriate compensatory therapeutic measures should be initiated as clinically indicated.
Due to the content of glucose and lactate in the solution, it may lead to hyperglycemia, especially in diabetic patients. Blood glucose levels should be monitored regularly. In case of hyperglycemia, it may be necessary to administer a dextrose-free replacement solution or dialysate. Other corrective measures may be necessary to maintain the desired glycemic control.
Prismasol contains sodium hydrogen carbonate (bicarbonate) and lactate (a precursor of bicarbonate) that can influence acid-base balance. If metabolic alkalosis develops or worsens during treatment with the solution, it may be necessary to reduce the administration rate or stop administration.
Before and during treatment, close monitoring of electrolyte and acid-base balance should be performed throughout the procedure.
In case of fluid imbalance, the clinical situation should be carefully controlled and fluid balance should be corrected as necessary.
Method of Administration
Intravenous and for hemodialysis. Prismasol, when used as a replacement solution, is administered within the circuit before the hemofilter (pre-dilution) or after the hemofilter (post-dilution).
Posology
The volume and rate of use of Prismasol will depend on the blood electrolyte concentration, acid-base balance, and the patient's overall clinical condition. The administration schedule (dose, perfusion rate, and cumulative volume) of Prismasol should be established by a doctor.
The flow rates used for the replacement solution in hemofiltration and hemodiafiltration are:
Adults: 500 - 3000 ml/h
The flow rates used for the dialysis solution (dialysate) in continuous hemodialysis and continuous hemodiafiltration are:
Adults: 500 - 2500 ml/h
Normally, the flow rates used in adults are approximately 2000 ml/h to 2500 ml/h, which corresponds to a daily fluid volume of approximately 48 to 60 liters.
Pediatric Population
The flow rate ranges for the replacement solution in hemofiltration and hemodiafiltration and for the dialysis solution (dialysate) in continuous hemodialysis are:
Children (from neonates to adolescents up to 18 years of age): 1000 to 2000 ml/h/1.73 m2.
Flow rates of up to 4000 ml/h/1.73 m2 may be necessary, especially in small children (≤10 kg). The absolute flow rate (in ml/h) in the pediatric population should not generally exceed the maximum flow rate in adults.
Handling Instructions
The electrolyte solution (small Compartment A) is added to the buffer solution (large Compartment B) after breaking the breakable valve, just before use, to obtain the reconstituted solution.
Use only with suitable extracorporeal renal replacement therapy equipment.
Aseptic technique should be followed during the entire process of handling and administration to the patient.
Use only if the overbag is intact, all seals are intact, the breakable valve is not broken, and the solution is transparent. Squeeze the bag firmly to ensure there are no leaks. If leaks are observed, discard the solution immediately, as sterility cannot be guaranteed.
The large compartment (B) is equipped with an injection port for adding other medications that may be necessary once the solution is reconstituted. It is the responsibility of the doctor to assess the compatibility of a medication added to Prismasol, checking for any color change and/or precipitation. Before adding a medication, verify that it is soluble and stable in water at the pH of Prismasol (the pH of the reconstituted solution is between 7.0 and 8.5). Additives may not be compatible. The instructions for use of the added medication should be consulted.
Remove any liquid from the injection port, put the bag in an inverted position, add the medication through the injection port, and mix perfectly. The solution should be administered immediately. The introduction and mixing of additives should always be done before connecting the solution bag to the extracorporeal circuit.
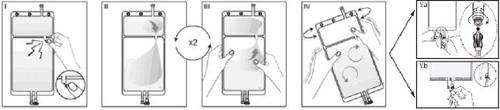
IRemove the overbag from the bag immediately before use and discard any other packaging material. Open the seal by breaking the breakable valve located between the two compartments of the bag. The valve will remain inside the bag. (See figure I below).
IIEnsure that all the liquid from the small Compartment A is transferred to the large Compartment B. (See figure II below).
IIIRinse twicethe small Compartment A by forcing the mixed solution to return to this compartment and then again to the large Compartment B (See figure III below).
IVOnce the small Compartment A is empty, shake the large Compartment B to mix its contents completely. The solution is now ready to use and can be hung on the equipment. (See figure IV below).
VThe dialysis or replacement line can be connected to either of the two access ports.
V.aIf the luer connector is used, remove the cap by twisting and pulling, and connect the male luer connector of the dialysis or replacement line to the female luer receptor of the bag by pushing and twisting. Ensure that the connection is secure and tightened. The connection will open. Check that the liquid flows freely. (See figure Va below).
If the dialysis or replacement line is disconnected from the luer connector, the connector will close, and the flow of the solution will stop. The luer port is a needle-free port that can be cleaned.
V.bIf the injection access is used, first remove the cap. The injection port is a port that can be disinfected with a swab. Insert the spike through the rubber wall. Verify that the solution flows freely. (See figure Vb below).
The solution should be used immediately after removing the overbag. If not, the reconstituted solution should be used within 24 hours, including the duration of treatment after adding the electrolyte solution to the buffer solution.
The reconstituted solution is for single use. Discard any unused solution immediately.
The disposal of unused medicinal products and all materials that have been in contact with them should be done in accordance with local regulations.
PVC FORMAT WITH BREAKABLE VALVE
What Prismasol contains
The active ingredients are.
Before reconstitution:
1000 ml of Electrolyte Solution (Small Compartment A) contains:
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose 22.000 g
(S)-Lactic acid 5.400 g
1000 ml of Buffer Solution (Large Compartment B) contains:
Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
Potassium chloride 0.314 g
After reconstitution:
The solution from Compartment A (250 ml) and Compartment B (4750 ml) is mixed to produce a reconstituted solution (5000 ml) with the following composition:
mmol/l | mEq/l | |
Calcium Ca2+ | 1.75 | 3.50 |
Magnesium Mg2+ | 0.50 | 1.00 |
Sodium Na+ | 140.00 | 140.00 |
Chloride Cl- | 113.50 | 113.50 |
Lactate | 3.00 | 3.00 |
Sodium hydrogen carbonate HCO3- | 32.00 | 32.00 |
Potassium K+ | 4.00 | 4.00 |
Glucose | 6.10 | |
Theoretical osmolality: | 301 mOsm/l |
The other components are:carbon dioxide (E-290), water for injectable preparations.
pH of the reconstituted solution: 7.0-8.5
Appearance of the product and container contents
Prismasol is presented in a bicompartmental bag, containing the small Compartment A with the electrolyte solution and the large Compartment B with the buffer solution. The final reconstituted solution is obtained after breaking the breakable valve and mixing both solutions. The reconstituted solution is transparent and slightly yellow. Each bag (A+B) contains 5000 ml of solution for hemodialysis and hemofiltration. The bag is covered by a transparent overbag.
Each box contains two bags and a leaflet.
Marketing Authorization Holder:
Vantive Belgium SRL
Boulevard d'Angleterre 2
1420 Braine-l'Alleud
Belgium
Manufacturer:
Bieffe Medital S.p.A.
Via Stelvio 94
23035 Sondalo (SO)
Italy
Or
Vantive Manufacturing Limited
Moneen Road,
Castlebar
County Mayo
F23 XR63
Ireland
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany, Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Slovakia, Slovenia, Estonia, Spain, Finland, France, Greece, Netherlands, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Portugal, Romania, Sweden, United Kingdom (Northern Ireland): Prismasol 4
For further information on this medicinal product, please contact the local representative of the Marketing Authorization Holder:
Vantive Health, S.L.
Industrial Estate Sector 14
C/ Pouet de Camilo, 2
46394 Ribarroja del Turia (Valencia)
Spain
Date of last revision of this leaflet: 03/2018
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es
-----------------------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only
Prismasol 4 mmol/l Potassium Solution for Hemodialysis and Hemofiltration
Precautions
The instructions for use and handling of Prismasol must be followed accurately.
The solutions from the two compartments must be mixed before use.
The use of contaminated hemofiltration and hemodialysis solutions can cause sepsis, shock, and conditions that can lead to death.
Prismasol can be warmed to 37°C to improve patient comfort. The warming of the solution before use must be done before reconstitution, using only dry heat. The solutions must not be warmed in water or in the microwave. The solution must be visually inspected before administration to detect the presence of particles and any possible color change, when the solution and container allow it. Do not administer if the solution is not transparent or if the seal is not intact.
Prismasol is a solution that contains potassium. The solution should not be administered to patients with hyperkalemia. Before and during hemofiltration and/or hemodialysis, the blood potassium concentration should be monitored.
If hyperkalemia occurs once treatment has started, the addition of potassium sources that affect concentrations should be assessed. When the solution is used as a replacement solution, the perfusion rate should be decreased and it should be confirmed that the desired potassium concentration has been reached. If hyperkalemia is not resolved, perfusion should be stopped immediately.
If hyperkalemia develops when using the solution as dialysate, it may be necessary to administer a potassium-free dialysate to increase potassium elimination.
The concentration of inorganic phosphates should be measured regularly. In the event that blood inorganic phosphate levels are low, they should be restored. A phosphate concentration of up to 1.2 mmol/l can be added to the solution. If phosphate is added to the bag, sodium phosphate should be used.
Although no cases of severe hypersensitivity reactions to corn with Prismasol have been reported, solutions containing glucose derived from hydrolyzed corn starch should not be used in patients with known allergy to corn or corn-derived products.
If signs or symptoms of suspected hypersensitivity reactions develop, administration should be interrupted immediately. Appropriate compensatory therapeutic measures should be initiated as clinically indicated.
Due to the content of glucose and lactate in the solution, it may lead to hyperglycemia, especially in diabetic patients. Blood glucose levels should be monitored regularly. In case of hyperglycemia, it may be necessary to administer a dextrose-free replacement solution or dialysate. Other corrective measures may be necessary to maintain the desired glycemic control.
Prismasol contains sodium hydrogen carbonate (bicarbonate) and lactate (a precursor of bicarbonate) that can influence acid-base balance. If metabolic alkalosis develops or worsens during treatment with the solution, it may be necessary to reduce the administration rate or stop administration.
Before and during treatment, close monitoring of electrolyte and acid-base balance should be performed throughout the procedure.
In case of fluid imbalance, the clinical situation should be carefully controlled and fluid balance should be corrected as necessary.
Method of Administration
Intravenous and for hemodialysis. Prismasol, when used as a replacement solution, is administered within the circuit before the hemofilter (pre-dilution) or after the hemofilter (post-dilution).
Posology
The volume and rate of use of Prismasol will depend on the blood electrolyte concentration, acid-base balance, and the patient's overall clinical condition. The administration schedule (dose, perfusion rate, and cumulative volume) of Prismasol should be established by a doctor.
The flow rates used for the replacement solution in hemofiltration and hemodiafiltration are:
Adults: 500 - 3000 ml/h
The flow rates used for the dialysis solution (dialysate) in continuous hemodialysis and continuous hemodiafiltration are:
Adults: 500 - 2500 ml/h
Normally, the flow rates used in adults are approximately 2000 ml/h to 2500 ml/h, which corresponds to a daily fluid volume of approximately 48 to 60 liters.
Pediatric Population
The flow rate ranges for the replacement solution in hemofiltration and hemodiafiltration and for the dialysis solution (dialysate) in continuous hemodialysis are:
Children (from neonates to adolescents up to 18 years of age): 1000 to 2000 ml/h/1.73 m2.
Flow rates of up to 4000 ml/h/1.73 m2 may be necessary, especially in small children (≤10 kg). The absolute flow rate (in ml/h) in the pediatric population should not generally exceed the maximum flow rate in adults.
Handling Instructions
The electrolyte solution (small Compartment A) is added to the buffer solution (large Compartment B) after breaking the breakable valve, just before use, to obtain the reconstituted solution.
Use only with suitable extracorporeal renal replacement therapy equipment.
Aseptic technique should be followed during the entire process of handling and administration to the patient.
Use only if the overbag is intact, all seals are intact, the breakable valve is not broken, and the solution is transparent. Squeeze the bag firmly to ensure there are no leaks. If leaks are observed, discard the solution immediately, as sterility cannot be guaranteed.
The large compartment (B) is equipped with an injection port for adding other medications that may be necessary once the solution is reconstituted. It is the responsibility of the doctor to assess the compatibility of a medication added to Prismasol, checking for any color change and/or precipitation. Before adding a medication, verify that it is soluble and stable in water at the pH of Prismasol (the pH of the reconstituted solution is between 7.0 and 8.5). Additives may not be compatible. The instructions for use of the added medication should be consulted.
Remove any liquid from the injection port, put the bag in an inverted position, add the medication through the injection port, and mix perfectly. The solution should be administered immediately. The introduction and mixing of additives should always be done before connecting the solution bag to the extracorporeal circuit.
IRemove the overbag from the bag immediately before use and discard any other packaging material. Open the seal by breaking the breakable valve located between the two compartments of the bag. The valve will remain inside the bag. (See figure I below).
IIEnsure that all the liquid from the small Compartment A is transferred to the large Compartment B. (See figure II below).
IIIRinse twicethe small Compartment A by forcing the mixed solution to return to this compartment and then again to the large Compartment B (See figure III below).
IVOnce the small Compartment A is empty, shake the large Compartment B to mix its contents completely. The solution is now ready to use and can be hung on the equipment. (See figure IV below).
VThe dialysis or replacement line can be connected to either of the two access ports.
V.aIf the luer connector is used, remove the cap by twisting and pulling, and connect the male luer connector of the dialysis or replacement line to the female luer receptor of the bag by pushing and twisting. Ensure that the connection is secure and tightened. The connection will open. Check that the liquid flows freely. (See figure Va below).
If the dialysis or replacement line is disconnected from the luer connector, the connector will close, and the flow of the solution will stop. The luer port is a needle-free port that can be cleaned.
V.bIf the injection access is used, first remove the cap. The injection port is a port that can be disinfected with a swab. Insert the spike through the rubber wall. Verify that the solution flows freely. (See figure Vb below).
The solution should be used immediately after removing the overbag. If not, the reconstituted solution should be used within 24 hours, including the duration of treatment after adding the electrolyte solution to the buffer solution.
The reconstituted solution is for single use. Discard any unused solution immediately.
The disposal of unused medicinal products and all materials that have been in contact with them should be done in accordance with local regulations.

If hyperkalemia develops when using the solution as dialysate, it may be necessary to administer a potassium-free dialysate to increase potassium elimination.
The concentration of inorganic phosphates should be measured regularly. In the event that blood inorganic phosphate levels are low, they should be restored. A phosphate concentration of up to 1.2 mmol/l can be added to the solution. If phosphate is added to the bag, sodium phosphate should be used.
Although no cases of severe hypersensitivity reactions to corn with Prismasol have been reported, solutions containing glucose derived from hydrolyzed corn starch should not be used in patients with known allergy to corn or corn-derived products.
If signs or symptoms of suspected hypersensitivity reactions develop, administration should be discontinued immediately. Appropriate corrective therapeutic measures should be instituted according to clinical indications.
Due to the glucose and lactate content of the solution, it may lead to hyperglycemia, especially in diabetic patients. Blood glucose levels should be regularly monitored. In case of hyperglycemia, it may be necessary to administer a dextrose-free substitution or dialysis solution. To maintain desired glycemic control, other corrective measures may be necessary.
Prismasol contains sodium hydrogen carbonate (bicarbonate) and lactate (precursor of bicarbonate) that can influence acid-base balance. If metabolic alkalosis develops or worsens during treatment with the solution, it may be necessary to reduce the administration rate or discontinue administration.
Before and during treatment, close monitoring of electrolyte and acid-base balance should be performed throughout the procedure.
In case of fluid imbalance, the clinical situation should be carefully monitored and fluid balance should be corrected as necessary.
Method of administration
Intravenous and for hemodialysis. Prismasol, when used as a substitution solution, is administered within the circuit before the hemofilter (pre-dilution) or after the hemofilter (post-dilution).
Posology
The volume and rate of use of Prismasol will depend on the concentration of electrolytes in the blood, acid-base balance, and the patient's overall clinical condition. The administration schedule (dose, perfusion rate, and cumulative volume) of Prismasol should be established by a doctor.
The flow rates used for the substitution solution in hemofiltration and hemodiafiltration are:
Adults: 500 - 3000 ml/h
The flow rates used for the dialysis solution (dialysate) in continuous hemodialysis and continuous hemodiafiltration are:
Adults: 500 - 2500 ml/h
Normally, the flow rates used in adults are approximately 2000 ml/h to 2500 ml/h, which corresponds to a daily fluid volume of approximately 48 to 60 liters.
Pediatric population
The flow rate intervals for the substitution solution in hemofiltration and hemodiafiltration and for the dialysis solution (dialysate) in continuous hemodialysis are:
Children (from neonates to adolescents up to 18 years of age): 1000 to 2000 ml/h/1.73 m2.
Flow rates of up to 4000 ml/h/1.73 m2 may be necessary, especially in small children (≤10 kg). The absolute flow rate (in ml/h) in the pediatric population should not generally exceed the maximum flow rate in adults.
Handling instructions
The electrolyte solution (small compartment A) is added to the buffer solution (large compartment B) after breaking the breakable seal just before use to obtain the reconstituted solution.
Use only with suitable extracorporeal renal replacement therapy equipment.
Aseptic technique should be followed throughout the handling and administration process to the patient.
Use only if the overbag is intact, all seals are intact, the breakable seal is not broken, and the solution is transparent. Squeeze the bag firmly to ensure there are no leaks. If leaks are observed, discard the solution immediately as sterility cannot be guaranteed.
The large compartment (B) is equipped with an injection port for adding other medications that may be necessary once the solution is reconstituted. It is the responsibility of the doctor to judge the compatibility of a medication added to Prismasol, checking for any change in color and/or precipitation, insoluble complexes, or crystals.
Before adding a medication, verify that it is soluble and stable in water at the pH of Prismasol (the pH of the reconstituted solution is between 7.0 and 8.5). Additives may not be compatible. The instructions for use of the added medication should be consulted.
Remove any liquid from the injection port, put the bag in an inverted position, add the medication through the injection port, and mix perfectly. The solution should be administered immediately. The introduction and mixing of additives should always be performed before connecting the solution bag to the extracorporeal circuit.
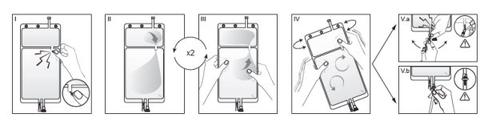
IRemove the overbag from the bag immediately before use and discard any other packaging material. Open the seal by breaking the breakable seal located between the two compartments of the bag. The seal will remain inside the bag. (See figure I below).
IIEnsure that all liquid from the small compartment A is transferred to the large compartment B. (See figure II below).
IIIClarify twicethe small compartment A by forcing the mixed solution to return to this compartment and then again to the large compartment B (See figure III below).
IVOnce the small compartment A is empty, shake the large compartment B to mix its contents completely. The solution is now ready to use and can be hung on the equipment. (See figure IV below).
VThe dialysis or substitution line can be connected to either of the two access ports.
V.aIf the luer access is used, remove the plug and connect the luer lock male connector on the dialysis or substitution line to the luer female receptor on the bag: do it firmly. Using your thumb and fingers, break the blue breakable seal at its base and move it back and forth. Do not use tools. Verify that the seal is completely separated and that the solution circulates freely. The seal will remain in the luer access during treatment. (See figure Va below).
V.bIf the injection access is used, remove the cap first. The injection port is a port that can be disinfected with a swab. Insert the spike through the rubber wall. Verify that the solution circulates freely. (See figure Vb below).
The solution should be used immediately after removing the overbag. If not, the reconstituted solution should be used within 24 hours, including the duration of treatment after adding the electrolyte solution to the buffer solution.
The reconstituted solution is for single use. Discard any unused solution immediately.
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
POLYOLEFIN FORMAT WITH VALVE
WHAT PRISMASOL CONTAINS
The active ingredients are:
Before reconstitution:
1000 ml of electrolyte solution (small compartment A) contains:
Calcium chloride dihydrate 5.145 g
Magnesium chloride hexahydrate 2.033 g
Glucose 22.000 g
(S)-Lactic acid 5.400 g
1000 ml of buffer solution (large compartment B) contains:
Sodium chloride 6.450 g
Sodium hydrogen carbonate 3.090 g
Potassium chloride 0.314 g
After reconstitution:
The solution from compartment A (250 ml) and compartment B (4750 ml) is mixed to produce a reconstituted solution (5000 ml) with the following composition:
mmol/l | mEq/l | |
Calcium Ca2+ | 1.75 | 3.50 |
Magnesium Mg2+ | 0.50 | 1.00 |
Sodium Na+ | 140.00 | 140.00 |
Chloride Cl- | 113.50 | 113.50 |
Lactate | 3.00 | 3.00 |
Hydrogen carbonate HCO3- | 32.00 | 32.00 |
Potassium K+ | 4.00 | 4.00 |
Glucose | 6.10 | |
Theoretical osmolality: | 301 mOsm/l |
Other components are:carbon dioxide (E-290), water for injectable preparations.
pH of the reconstituted solution: 7.0-8.5
Appearance of the product and packaging content
Prismasol is presented in a bicompartmental bag, containing the small compartment A the electrolyte solution and the large compartment B the buffer solution. The final reconstituted solution is obtained after breaking the peelable seal and mixing both solutions. The reconstituted solution is transparent and slightly yellow. Each bag (A+B) contains 5000 ml of solution for hemofiltration and hemodialysis. The bag is covered by a transparent overbag.
Each box contains two bags and a leaflet.
Marketing authorization holder:
Vantive Belgium SRL
Boulevard d'Angleterre 2
1420 Braine-l'Alleud
Belgium
Manufacturer:
Bieffe Medital S.p.A.
Via Stelvio 94
23035 Sondalo (SO)
Italy
Or
Vantive Manufacturing Limited Moneen Road,
Castlebar
County Mayo
F23 XR63
Ireland
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany, Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Slovakia, Slovenia, Estonia, Spain, Finland, France, Greece, Netherlands, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Portugal, Romania, Sweden, United Kingdom (Northern Ireland): Prismasol 4
Further information about this medicinal product can be obtained from the local representative of the marketing authorization holder:
Vantive Health, S.L.
Polígono industrial sector 14
C/ Pouet de Camilo nº2
46394 Ribarroja del Turia
Valencia
Spain
Date of last revision of this leaflet: 03/2018
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es
-----------------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals
Prismasol 4 mmol/l Potassium solution for hemodialysis and hemofiltration
Precautions
The instructions for use and handling of Prismasol should be followed accurately.
The solutions from the two compartments must be mixed before use.
The use of contaminated hemofiltration and hemodialysis solutions can cause sepsis, shock, and conditions that can lead to death.
Prismasol can be warmed to 37°C to improve patient comfort. Warming of the solution before use should be done before reconstitution using only dry heat. The solutions should not be warmed in water or in the microwave. The solution should be visually inspected before administration to detect the presence of particles and any change in color, when the solution and container allow. Do not administer if the solution is not transparent or if the seal is not intact.
Prismasol is a solution that contains potassium. The solution should not be administered to patients with hyperkalemia. Before and during hemofiltration and/or hemodialysis, blood potassium concentration should be monitored.
If hyperkalemia occurs once treatment has started, the addition of potassium sources that affect potassium concentrations should be assessed. When the solution is used as a substitution solution, the perfusion rate should be decreased and it should be confirmed that the desired potassium concentration has been achieved. If hyperkalemia is not resolved, perfusion should be stopped immediately.
If hyperkalemia develops when the solution is used as dialysate, it may be necessary to administer a potassium-free dialysate to increase potassium elimination.
The concentration of inorganic phosphates should be measured regularly. In the event that blood inorganic phosphate levels are low, they should be restored. A phosphate concentration of up to 1.2 mmol/l can be added to the solution. If phosphate is added to the bag, sodium phosphate should be used.
Although no cases of severe hypersensitivity reactions to corn with Prismasol have been reported, solutions containing glucose derived from hydrolyzed corn starch should not be used in patients with known allergy to corn or corn-derived products.
If signs or symptoms of suspected hypersensitivity reactions develop, administration should be discontinued immediately. Appropriate corrective therapeutic measures should be instituted according to clinical indications.
Due to the glucose and lactate content of the solution, it may lead to hyperglycemia, especially in diabetic patients. Blood glucose levels should be regularly monitored. In case of hyperglycemia, it may be necessary to administer a dextrose-free substitution or dialysis solution. To maintain desired glycemic control, other corrective measures may be necessary.
Prismasol contains sodium hydrogen carbonate (bicarbonate) and lactate (precursor of bicarbonate) that can influence acid-base balance. If metabolic alkalosis develops or worsens during treatment with the solution, it may be necessary to reduce the administration rate or discontinue administration.
Before and during treatment, close monitoring of electrolyte and acid-base balance should be performed throughout the procedure.
In case of fluid imbalance, the clinical situation should be carefully monitored and fluid balance should be corrected as necessary.
Method of administration
Intravenous and for hemodialysis. Prismasol, when used as a substitution solution, is administered within the circuit before the hemofilter (pre-dilution) or after the hemofilter (post-dilution).
Posology
The volume and rate of use of Prismasol will depend on the concentration of electrolytes in the blood, acid-base balance, and the patient's overall clinical condition. The administration schedule (dose, perfusion rate, and cumulative volume) of Prismasol should be established by a doctor.
The flow rates used for the substitution solution in hemofiltration and hemodiafiltration are:
Adults: 500 - 3000 ml/h
The flow rates used for the dialysis solution (dialysate) in continuous hemodialysis and continuous hemodiafiltration are:
Adults: 500 - 2500 ml/h
Normally, the flow rates used in adults are approximately 2000 ml/h to 2500 ml/h, which corresponds to a daily fluid volume of approximately 48 to 60 liters.
Pediatric population
The flow rate intervals for the substitution solution in hemofiltration and hemodiafiltration and for the dialysis solution (dialysate) in continuous hemodialysis are:
Children (from neonates to adolescents up to 18 years of age): 1000 to 2000 ml/h/1.73 m2.
Flow rates of up to 4000 ml/h/1.73 m2 may be necessary, especially in small children (≤10 kg). The absolute flow rate (in ml/h) in the pediatric population should not generally exceed the maximum flow rate in adults.
Handling instructions
The electrolyte solution (small compartment A) is added to the buffer solution (large compartment B) after breaking the peelable seal just before use to obtain the reconstituted solution.
Use only with suitable extracorporeal renal replacement therapy equipment.
Aseptic technique should be followed throughout the handling and administration process to the patient.
Use only if the overbag is intact, all seals are intact, the peelable seal is not broken, and the solution is transparent. Squeeze the bag firmly to ensure there are no leaks. If leaks are observed, discard the solution immediately as sterility cannot be guaranteed.
The large compartment (B) is equipped with an injection port for adding other medications that may be necessary once the solution is reconstituted. It is the responsibility of the doctor to judge the compatibility of a medication added to Prismasol, checking for any change in color and/or precipitation, insoluble complexes, or crystals.
Before adding a medication, verify that it is soluble and stable in water at the pH of Prismasol (the pH of the reconstituted solution is between 7.0 and 8.5). Additives may not be compatible. The instructions for use of the added medication should be consulted.
Remove any liquid from the injection port, put the bag in an inverted position, add the medication through the injection port, and mix perfectly. The solution should be administered immediately. The introduction and mixing of additives should always be performed before connecting the solution bag to the extracorporeal circuit.
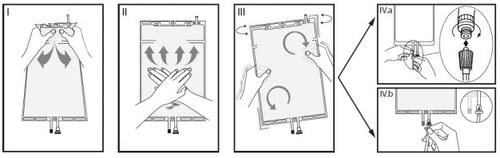
Different compartments. Hold the small compartment with both hands and squeeze until the detachable sealed wall between the two compartments opens. (See figure I below).
IIPress the large compartment with both hands until the sealed wall between the two compartments is completely open. (See figure II below).
IIIMake sure the solutions are completely mixed by gently shaking the bag. The solution is now ready to use and the bag can be hung on the equipment. (See figure III below).
IVThe dialysis or substitution line can be connected to either of the two access ports.
IV.aIf the luer connector is used, remove the plug by a twisting and pulling motion and connect the male luer connector of the dialysis or substitution line to the female luer receptor of the bag by a pushing and twisting motion. Ensure that the connection is secure and tightened. The connection will open. Check that the liquid circulates freely. (See figure IVa below).
If the dialysis or substitution line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. The luer port is a needleless port that can be cleaned.
IV.bIf the injection access is used, first remove the capsule. The injection port is a port that can be disinfected with a swab. Insert the spike through the rubber wall. Verify that the solution circulates freely. (See figure IVb below).
The solution should be used immediately after removing the overbag. If not, the reconstituted solution should be used within 24 hours, including the duration of treatment after adding the electrolyte solution to the buffer solution.
The reconstituted solution is for single use. Discard any unused solution immediately.
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PRISMASOL 4 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATIONDosage form: HEMOFILTRATION, 2 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, 4 mmol potassium/lActive substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription requiredDosage form: HEMOFILTRATION, -Active substance: HemofiltratesManufacturer: Nikkiso BelgiumPrescription required
Online doctors for PRISMASOL 4 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION
Discuss questions about PRISMASOL 4 mmol/L POTASSIUM SOLUTION FOR HEMODIALYSIS AND HEMOFILTRATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















