
POMBILITI 105 mg POWDER FOR CONCENTRATE FOR PERFUSION SOLUTION

How to use POMBILITI 105 mg POWDER FOR CONCENTRATE FOR PERFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Pombiliti 105 mg powder for concentrate for solution for infusion
cipaglucosidase alfa
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the package leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start receiving this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Pombiliti and what is it used for
- What you need to know before you receive Pombiliti
- How Pombiliti is administered
- Possible side effects
- Storage of Pombiliti
- Contents of the pack and other information
1. What is Pombiliti and what is it used for
Pombiliti is a type of "enzyme replacement therapy" (ERT) that is indicated for adults with late-onset Pompe disease. It contains the active substance called "cipaglucosidase alfa".
What it is used for
Pombiliti is always used together with another medicine called miglustat 65 mg hard capsules. It is very important that you also read the package leaflet of miglustat 65 mg hard capsules.
If you have any questions about these medicines, ask your doctor or pharmacist.
How Pombiliti works
People with Pompe disease have low levels of an enzyme called acid alpha-glucosidase (GAA). This enzyme helps regulate the levels of glycogen (a type of carbohydrate) in the body.
In Pompe disease, large amounts of glycogen accumulate in the muscles throughout the body. This prevents the muscles from working properly, for example, the muscles that help you walk, those that facilitate breathing in the lungs, and the heart muscle.
Pombiliti enters the muscle cells that are affected by Pompe disease. Once inside the cells, the medicine works like GAA, helping to break down glycogen and regulate its levels.
2. What you need to know before you receive Pombiliti
Do not receive Pombiliti
- If you have ever had potentially life-threatening hypersensitivity reactions to the following:
- cipaglucosidase alfa
- miglustat
- any of the other ingredients of this medicine (listed in section 6).
- If a previous infusion had to be interrupted and could not be resumed due to potentially life-threatening hypersensitivity reactions.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before starting to use Pombiliti.
Talk to your doctor or nurse immediatelyif any of these situations apply to you, or if you think they might, or if you have ever had any of these reactions with another enzyme replacement therapy (ERT):
- allergic reactions, including anaphylaxis (a severe allergic reaction) - see section 4 below for symptoms of potentially life-threatening reactions;
- infusion-related reaction while receiving the medicine or in the hours following, see section 4 below for symptoms of potentially life-threatening reactions.
Tell your doctor if you have a history of any heart or lung disease. These diseases may worsen during or immediately after the infusion of Pombiliti. Tell your doctor or nurse immediately if you experience difficulty breathing, coughing, rapid or irregular heartbeat, or any other effect of these diseases.
Also, inform your doctor if you experience swelling in your legs or generalized swelling of the body, severe skin rash, or foamy urine when eliminating fluids. Your doctor will decide whether the infusion of Pombiliti should be interrupted and will provide you with the appropriate medical treatment. Similarly, your doctor will decide whether you can continue to receive Pombiliti.
Medicines before treatment
Your doctor may give you other medicines before treatment with Pombiliti, for example:
- antihistamines and corticosteroids to prevent or mitigate infusion-related reactions;
- antipyretics to reduce fever.
Children and adolescents
This medicine must not be given to patients under 18 years of age, because the effects of Pombiliti in combination with miglustat are unknown in this age group.
Other medicines and Pombiliti
Tell your doctor or nurse if you are using, have recently used, or might use any other medicines, including those bought without a prescription and herbal medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, you must not receive this medicine, but consult your doctor or pharmacist immediately before using it.
There are no data on the use of Pombiliti in combination with miglustat during pregnancy.
- If you are pregnant, you must not receive Pombiliti and/or take miglustat 65 mg hard capsules. Inform your doctor immediately if you become pregnant, think you may be pregnant, or plan to become pregnant. There may be risks for the unborn baby.
- Pombiliti in combination with miglustat must not be given to women who are breastfeeding. A decision must be made whether to stop breastfeeding or stop the treatment.
Contraception and fertility
Women of childbearing potential must use effective contraceptive methods during and 4 weeks after the end of treatment with both medicines.
Driving and using machines
You may experience dizziness, drowsiness, or low blood pressure (hypotension) after receiving Pombiliti or the medicines before treatment. In this case, do not drive or use tools or machines.
Pombiliti contains sodium
This medicine contains 10.5 mg of sodium (main component of cooking/table salt) per vial. This is equivalent to 0.52% of the maximum recommended daily sodium intake for an adult.
3. How Pombiliti is administered
Pombiliti is administered by a doctor or nurse. It is given as a drip into a vein. This method of administration is called intravenous infusion.
Ask your doctor if you want to be treated at home. Your doctor will decide, after evaluation, whether it is safe for you to receive the infusion of Pombiliti at your home. If you experience any side effects during an infusion of Pombiliti, the healthcare professional administering it may stop the infusion and start the corresponding medical treatment.
Pombiliti must be used in combination with miglustat. You can only use miglustat 65 mg hard capsules with cipaglucosidase alfa. DO NOTuse miglustat 100 mg hard capsules (different medicine). Regarding the recommended dose, follow your doctor's instructions and read the package leaflet of miglustat 65 mg hard capsules.
How much Pombiliti is administered
The amount of medicine you receive is based on your weight. The recommended dose is 20 mg per kilogram of body weight.
When Pombiliti is administered and for how long
- You will receive treatment with Pombiliti once every two weeks. Miglustat 65 mg hard capsules are taken on the same day as the administration of Pombiliti. See the package leaflet of miglustat 65 mg hard capsules for information on how to take miglustat.
- The infusion of cipaglucosidase alfa must start 1 hour after taking miglustat 65 mg hard capsules.
- In case of delay, the start of the infusion must not exceed 3 hours from the intake of miglustat.
- The infusion of cipaglucosidase alfa lasts approximately 4 hours.
Figure 1. Chronological development of doses
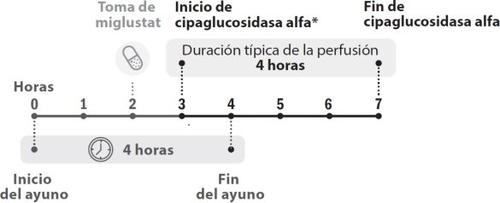
- The infusion of cipaglucosidase alfa must start 1 hour after taking the miglustat capsules. In case of delay in the infusion, the start of the infusion must not exceed 3 hours from the intake of miglustat.
Switching from another enzyme replacement therapy (ERT)
If you are currently receiving another ERT:
- Your doctor will tell you when you should stop the other ERT before starting Pombiliti.
- Tell your doctor when you received the last dose.
If you receive more Pombiliti than you should
If you have difficulty breathing, feel swollen or inflamed, or notice your heart racing, you may have been given too much Pombiliti; inform your doctor immediately. Excess infusion rate of Pombiliti could cause symptoms due to excess fluid in the body, such as difficulty breathing, high heart rate, or generalized swelling of the body.
If you miss a dose of Pombiliti
If you have missed an infusion, contact your doctor or nurse as soon as possible to schedule an appointment and receive Pombiliti in combination with miglustat 24 hours after the last intake of miglustat.
If you stop treatment with Pombiliti
Talk to your doctor if you want to stop treatment with Pombiliti. The symptoms of your disease may worsen if you stop treatment.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Pombiliti is used together with miglustat, and either of these medicines may cause side effects. Side effects have been observed mainly in patients during the infusion of Pombiliti (infusion-related reactions) or shortly after. Inform your doctor immediately if you experience an infusion-related reaction or an allergic reaction. Some of these reactions can be serious and potentially life-threatening. Your doctor may give you medicines before the infusion to prevent these reactions.
Infusion-related reactions
Most infusion-related reactions are mild or moderate. The symptoms of an infusion-related reaction include difficulty breathing, swelling, fever, chills, dizziness, flushing, and itching of the skin and rash.
Allergic reactions
Allergic reactions can cause symptoms such as rash on any part of the body, swelling of the eyes, prolonged difficulty breathing, coughing, swelling of the lips, tongue, or throat, itching of the skin, and hives.
Very common(may affect more than 1 in 10 people)
- Headache
Common(may affect up to 1 in 10 people)
- Cough
- Sudden reddening of the face, neck, or upper chest
- Chest pain
- Rash, itching
- High blood pressure
- Sweating
- Abdominal swelling
- Flatulence or gas
- Diarrhea, loose stools
- Vomiting
- Nausea
- Fever or chills
- Hives
- Swelling or pain at the infusion site
- Cramps, muscle pain or weakness
- Tremors in one or more parts of the body
- Increased sweating
- Pain
- Altered sense of taste
- Feeling of constant tiredness or sleepiness
- Difficulty breathing
Uncommon(may affect up to 1 in 100 people)
- Difficult breathing that causes coughing, wheezing when breathing, and feeling of lack of air (asthma)
- Allergic reaction
- Swelling of the hands, feet, ankles, legs
- Swelling of the face
- Indigestion
- Stomach pain
- Feeling of constant tiredness
- Sore throat
- Painful or abnormal contractions of the throat
- Mouth irritation
- Pain in the mouth or discomfort in the back of the mouth
- Pain in the cheeks, gums, lips, chin
- Loss of strength and energy, feeling of weakness
- Malaise, general feeling of lethargy
- Burning sensation
- Scratches or lesions on the skin
- Body temperature changes
- Decrease in a type of white blood cell (detected in blood tests)
- Drowsiness
- Dizziness
- Joint pain
- Pain in the area between the hip and the ribs
- Muscle fatigue
- Increased muscle stiffness
- Inability to maintain balance
- Low blood pressure
- Feeling of being about to faint
- Pain on one or both sides of the head, stabbing pain, aura, pain in the eyes, sensitivity to light (migraine)
- Spots on the skin
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Pombiliti
Your doctor, pharmacist, or nurse is responsible for storing this medicine and disposing of opened vials properly. This information is intended only for healthcare professionals.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial and carton after "EXP". The expiry date is the last day of the month shown.
Unopened vials: Store in a refrigerator (between 2°C and 8°C). Keep the vial in the outer packaging to protect it from light.
After dilution, it is recommended to use immediately. However, the storage of the intravenous infusion bag with Pombiliti has been demonstrated for 6 hours at a temperature between 20°C and 25°C and for 24 hours at a temperature between 2°C and 8°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Pombiliti composition
The active ingredient is cipaglucosidase alfa. One vial contains 105 mg of cipaglucosidase alfa. After reconstitution, the solution contained in the vial contains 15 mg of cipaglucosidase alfa per milliliter. A final concentration of cipaglucosidase alfa diluted in the intravenous infusion bag of 0.5 mg/ml to 4 mg/ml is recommended.
The other components are:
- Sodium citrate dihydrate (E331)
- Citric acid monohydrate (E330)
- Mannitol (E421)
- Polysorbate 80 (E433)
Product appearance and container contents
Pombiliti is a white to slightly yellow powder. After reconstitution, it is a transparent to opalescent solution, between colorless and slightly yellow, without foreign particles and practically free of white to translucent particles. The reconstituted solution must be diluted subsequently in an intravenous infusion bag.
Pombiliti is a powder for concentrate for solution for infusion in a vial.
Packs of 1, 10 or 25 vials
Only some pack sizes may be marketed.
Marketing authorization holder
Amicus Therapeutics Europe Limited
Block 1, Blanchardstown Corporate Park
Ballycoolin Road
Blanchardstown, Dublin
D15 AKK1
Ireland
Tel.: +353 (0) 1 588 0836
Fax: +353 (0) 1 588 6851
Email: [email protected]
Manufacturer
Manufacturing Packaging Farmaca (MPF) B.V.
Neptunus 12, Heerenveen, 8448CN, Netherlands
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
België/Belgique/Belgien Amicus Therapeutics Europe Limited Tél/Tel: (+32) 0800 89172 email: [email protected] | Lietuva Amicus Therapeutics Europe Limited Tel: (+370) 8800 33167 El. paštas: [email protected] |
| Luxembourg/Luxemburg Amicus Therapeutics Europe Limited Tél/Tel: (+352) 800 27003 email: [email protected] |
Ceská republika Amicus Therapeutics Europe Limited Tel.: (+420) 800 142 207 email: [email protected] | Magyarország Amicus Therapeutics Europe Limited Tel.: (+36) 06 800 21202 email: [email protected] |
Danmark Amicus Therapeutics Europe Limited Tlf.: (+45) 80 253 262 email: [email protected] | Malta Amicus Therapeutics Europe Limited Tel: (+356) 800 62674 email: [email protected] |
Deutschland Amicus Therapeutics GmbH Tel: (+49) 0800 000 2038 E-Mail: [email protected] | Nederland Amicus Therapeutics BV Tel: (+31) 20 235 8510/(+31) 0800 022 8399 email: [email protected] |
Eesti Amicus Therapeutics Europe Limited Tel: (+372) 800 0111 911 e-post: [email protected] | Norge Amicus Therapeutics Europe Limited Tlf: (+47) 800 13837 e-post: [email protected] |
Ελλάδα Amicus Therapeutics Europe Limited Τηλ: (+30) 00800 126 169 email: [email protected] | Österreich Amicus Therapeutics Europe Limited Tel: (+43) 0800 909 639 email: [email protected] |
España Amicus Therapeutics S.L.U. Tel: (+34) 900 941 616 email: [email protected] | Polska Amicus Therapeutics Europe Limited Tel.: (+48) 0080 012 15475 email: [email protected] |
France Amicus Therapeutics SAS Tél: (+33) 0 800 906 788 email: [email protected] | Portugal Amicus Therapeutics Europe Limited Tel: (+351) 800 812 531 email: [email protected] |
Hrvatska Amicus Therapeutics Europe Limited Tel: (+358) 0800 222 452 e-pošta: [email protected] | Ireland Amicus Therapeutics Europe Limited Tel: (+353) 1800 936 230 email: [email protected] |
România Amicus Therapeutics Europe Limited Tel.: (+40) 0808 034 288 email: [email protected] | Slovenija Amicus Therapeutics Europe Limited Tel.: (+386) 0800 81794 e-pošta: [email protected] |
Ísland Amicus Therapeutics Europe Limited Sími: (+354) 800 7634 Netfang: [email protected] | Slovenská republika Amicus Therapeutics Europe Limited Tel: (+421) 0800 002 437 email: [email protected] |
Italia Amicus Therapeutics S.r.l. Tel: (+39) 800 795 572 email: [email protected] | Suomi/Finland Amicus Therapeutics Europe Limited Puh/Tel: (+358) 0800 917 780 sähköposti/email: [email protected] |
Κύπρος Amicus Therapeutics Europe Limited Τηλ: (+357) 800 97595 email: [email protected] | Sverige Amicus Therapeutics Europe Limited Tfn: (+46) 020 795 493 e-post: [email protected] |
Latvija Amicus Therapeutics Europe Limited Tel: (+371) 800 05391 e-pasts: [email protected] | United Kingdom (Northern Ireland) Amicus Therapeutics, UK Limited Tel: (+44) 08 0823 46864 email: [email protected] |
Date of last revision of this prospectus
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
This information is intended solely for healthcare professionals:
Instructions for use: reconstitution, dilution, and administration
Pombiliti must be reconstituted with water for injectable preparations and then diluted in a sodium chloride 9 mg/ml (0.9%) solution for injectable preparations and administered by intravenous infusion. Reconstitution and dilution must be performed in accordance with good clinical practice standards, particularly with regard to asepsis.
Since this medicinal product is a protein, particles may form in the reconstituted solution and in the final diluted infusion bag. Therefore, a 0.2-micron low-protein-binding in-line filter should be used for administration. The use of a 0.2-micron in-line filter has been shown to remove visible particles and does not result in apparent loss of protein or activity.
Determine the number of vials to be reconstituted based on the patient's dosage regimen (mg/kg) and remove the required vials from the refrigerator to reach room temperature (about 30 minutes). Each vial of Pombiliti is for single use only.
Use an aseptic technique.
Reconstitution
Reconstitute 105 mg per vial of Pombiliti in 7.2 ml of water for injectable preparations using a syringe with a needle diameter not exceeding 18 G. Add the water for injectable preparations drop by drop down the side of the vial and not directly onto the lyophilized powder. Tilt and gently rotate each vial. Do not invert, shake, or agitate the vial. The extraction volume is a transparent to opalescent solution, between colorless and slightly yellow, without foreign particles and practically free of white to translucent particles. Perform an immediate inspection of the reconstituted vials to verify that there are no particles or color alteration. If, during the immediate inspection, foreign particles other than those described above are observed or the reconstituted solution shows a color alteration, do not use it. The pH of the reconstituted solution is approximately 6.0.
After reconstitution, it is recommended to dilute the vials immediately (see below).
Dilution
Following the reconstitution described above, the reconstituted solution in the vial contains 15 mg of cipaglucosidase alfa per milliliter. The reconstituted volume allows for the exact extraction of 7.0 ml (equivalent to 105 mg) from each vial. The solution should be diluted subsequently as follows: using a syringe with a needle diameter not exceeding 18 G, slowly extract the reconstituted solution from each vial, including the volume below 7.0 ml from the partial vial, until the patient's dose is obtained. The recommended final concentration of cipaglucosidase alfa in the infusion bags is between 0.5 mg/ml and 4 mg/ml. Extract the air from the interior of the infusion bag.
Also, extract a volume equivalent to the sodium chloride 9 mg/ml (0.9%) injectable solution, which will be replaced by the volume of reconstituted Pombiliti. Slowly inject the reconstituted Pombiliti solution directly into the sodium chloride 9 mg/ml (0.9%) solution for injectable preparations. Gently invert or massage the infusion bag to mix the diluted solution. Do not shake or agitate the infusion bag excessively.
The final infusion solution should be administered as soon as possible after preparation.
Disposal of unused medicinal product and all materials that have come into contact with it should be done in accordance with local regulations.
Administration
Pombiliti infusion should start 1 hour after taking the miglustat capsules. In case of delayed infusion, its start should not exceed 3 hours from taking miglustat. The recommended dosage regimen for Pombiliti is 20 mg/kg body weight administered every two weeks by intravenous infusion.
Infusions should be administered gradually. It is recommended that the initial infusion rate be 1 mg/kg/h and gradually increase by 2 mg/kg/h every 30 minutes, if no signs of IR (infusion-related reactions) appear, until a maximum rate of 7 mg/kg/h is reached.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to POMBILITI 105 mg POWDER FOR CONCENTRATE FOR PERFUSION SOLUTIONDosage form: INJECTABLE INFUSION, 100 UActive substance: laronidaseManufacturer: Sanofi B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 30 mg/mlActive substance: cerliponase alfaManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE INFUSION, UnknownActive substance: imigluceraseManufacturer: Sanofi B.V.Prescription required
Online doctors for POMBILITI 105 mg POWDER FOR CONCENTRATE FOR PERFUSION SOLUTION
Discuss questions about POMBILITI 105 mg POWDER FOR CONCENTRATE FOR PERFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















