
POMALIDOMIDE TEVA 4 mg HARD CAPSULES

How to use POMALIDOMIDE TEVA 4 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information: Summary of Product Characteristics
Pomalidomide Teva 1 mg hard capsules
Pomalidomide Teva 2 mg hard capsules
Pomalidomide Teva 3 mg hard capsules
Pomalidomide Teva 4 mg hard capsules
pomalidomide
Pomalidomide Teva is expected to cause severe birth defects and can cause fetal death. Fetal death.
|
Read all of this summary of product characteristics carefully before you start taking this medicine because it contains important information for you.
- Keep this summary of product characteristics, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this summary of product characteristics. See section 4.
Contents of the summary of product characteristics
- What is Pomalidomide Teva and what is it used for
- What you need to know before you take Pomalidomide Teva
- How to take Pomalidomide Teva
- Possible side effects
- Storage of Pomalidomide Teva
- Contents of the pack and other information
1. What is Pomalidomide Teva and what is it used for
What is Pomalidomide Teva
Pomalidomide Teva contains the active substance "pomalidomide". This medicine is related to thalidomide and belongs to a group of medicines that affect the immune system (the body's natural defenses).
What is Pomalidomide Teva used for
Pomalidomide Teva is used to treat adults with a type of cancer called "multiple myeloma".
Pomalidomide Teva is used with:
- Other two medicinescalled "bortezomib" (a type of chemotherapy medicine) and "dexamethasone" (an anti-inflammatory medicine) in people who have received at least one other treatment, including lenalidomide.
U
- Another medicinecalled "dexamethasone" in people who have had their multiple myeloma get worse despite receiving at least two other types of treatments, including lenalidomide and bortezomib.
What is multiple myeloma
Multiple myeloma is a type of cancer that affects a specific type of white blood cell (called "plasma cells"). These cells grow out of control and accumulate in the bone marrow, damaging bones and kidneys.
Multiple myeloma usually has no cure. However, treatment can reduce the signs and symptoms of the disease or make them disappear for a period of time. When this happens, it is called a "response".
How Pomalidomide Teva works
Pomalidomide Teva works in several ways:
- stops the growth of myeloma cells;
- stimulates the immune system to attack cancer cells;
- stops the formation of blood vessels that feed cancer cells.
Benefit of using Pomalidomide Teva with bortezomib and dexamethasone
If Pomalidomide Teva is used with bortezomib and dexamethasone in people who have received at least one other treatment, it can stop the progression of multiple myeloma:
- On average, the combination of Pomalidomide Teva with bortezomib and dexamethasone prevented the return of multiple myeloma for up to 11 months compared to 7 months in patients who took only bortezomib and dexamethasone.
Benefit of using Pomalidomide Teva with dexamethasone
If Pomalidomide Teva is used with dexamethasone in people who have received at least two other treatments, it can stop the progression of multiple myeloma:
- On average, the combination of Pomalidomide Teva and dexamethasone prevented the return of multiple myeloma for up to 4 months compared to 2 months in patients who took dexamethasone alone.
2. What you need to know before you take Pomalidomide Teva
Do not take Pomalidomide Teva:
- if you are pregnant, think you may be pregnant, or plan to become pregnant, as Pomalidomide Teva is expected to be harmful to the fetus. (Men and women taking this medicine should read the section "Pregnancy, contraception, and breastfeeding – information for men and women" below);
- if you can become pregnant, unless you are using effective contraception (see "Pregnancy, contraception, and breastfeeding – information for men and women"). If you can become pregnant, your doctor will confirm that you have taken all necessary measures to prevent pregnancy and will provide you with this confirmation;
- if you are allergic to pomalidomide or any of the other ingredients of this medicine (listed in section 6). If you think you may be allergic, talk to your doctor.
If you are not sure if any of these situations apply to you, talk to your doctor, pharmacist, or nurse before taking Pomalidomide Teva.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before taking Pomalidomide Teva if:
- you have ever had blood clots in the past. During treatment with Pomalidomide Teva, you have a higher risk of developing blood clots in your veins or arteries. Your doctor may recommend additional treatments (e.g., warfarin) or reduce your dose of Pomalidomide Teva to reduce the risk of blood clots;
- you have ever had an allergic reaction, such as a skin rash, itching, swelling, dizziness, or breathing problems while taking medicines related to "thalidomide" or "lenalidomide";
- you have had a heart attack, have heart failure, have difficulty breathing, or if you are a smoker, have high blood pressure or high cholesterol levels;
- you have a high tumor burden in your body, including the bone marrow. This could lead to a condition where tumors break down and produce abnormal levels of chemicals in the blood, which can cause kidney failure. You may also experience irregular heartbeats. This condition is called tumor lysis syndrome;
- you have or have had neuropathy (nerve damage that causes tingling or pain in your feet or hands);
- you have or have had a hepatitis B virus infection. Treatment with Pomalidomide Teva may reactivate the hepatitis B virus in patients who are carriers of the virus, leading to the infection returning (recurrence). Your doctor should check if you have ever had a hepatitis B virus infection.
- you experience or have experienced in the past a combination of any of the following symptoms: rash on the face or generalized, skin redness, high fever, flu-like symptoms, swollen lymph nodes (symptoms of a severe skin reaction called drug reaction with eosinophilia and systemic symptoms or DRESS syndrome or hypersensitivity syndrome, toxic epidermal necrolysis [TEN] or Stevens-Johnson syndrome [SJS]). See also section 4 "Possible side effects".
It is important to note that patients with multiple myeloma treated with pomalidomide may develop other types of cancer, so your doctor should carefully evaluate the benefits and risks when prescribing this medicine.
At any time during or after treatment, tell your doctor or nurse immediately if you:
experience blurred vision, loss of vision, or double vision, difficulty speaking, weakness in one arm or one leg, a change in the way you walk or balance problems, persistent numbness, decreased sensitivity, or loss of sensitivity, memory loss, or confusion. These may be symptoms of a serious and potentially life-threatening brain disease called progressive multifocal leukoencephalopathy (PML). If you had any of these symptoms before starting treatment with Pomalidomide Teva, tell your doctor if you notice any change in these symptoms.
At the end of treatment, you must return all unused capsules to the pharmacist.
Pregnancy, contraception, and breastfeeding: information for men and women
You must follow the instructions in the Pomalidomide Teva Pregnancy Prevention Program. Men and women taking Pomalidomide Teva must not father a child or become pregnant. The reason is that pomalidomide is expected to be harmful to the fetus. You and your partner must use effective contraception while taking this medicine.
Women
Do not take Pomalidomide Teva if you are pregnant, think you may be pregnant, or plan to become pregnant. The reason is that this medicine is expected to be harmful to the fetus. Before starting treatment, you must tell your doctor if there is a possibility that you may become pregnant, even if you think this is unlikely.
If you can become pregnant:
- you must use effective contraception from at least 4 weeks before starting treatment, during treatment, and until at least 4 weeks after stopping treatment. Your doctor will advise you on the most suitable contraceptive methods;
- each time your doctor prescribes a prescription, they will ensure that you have understood the necessary measures to prevent pregnancy;
- your doctor will schedule pregnancy tests before treatment, at least every 4 weeks during treatment, and at least 4 weeks after stopping treatment.
If, despite preventive measures, you become pregnant:
- you must stop treatment immediately and inform your doctor immediately.
Breastfeeding
It is not known whether Pomalidomide Teva passes into human breast milk. Tell your doctor if you are breastfeeding or plan to breastfeed. Your doctor will advise you whether you can continue breastfeeding or should stop.
Men
Pomalidomide Teva passes into human semen.
- If your partner is pregnant or can become pregnant, you must use condoms during treatment and until 7 days after stopping treatment.
- If your partner becomes pregnant while you are taking Pomalidomide Teva, tell your doctor immediately. Your partner should also tell their doctor immediately.
You must not donate sperm or semen during treatment and until 7 days after stopping treatment.
Blood donation and blood tests
You must not donate blood during treatment and until 7 days after stopping treatment. Before starting treatment with Pomalidomide Teva and during treatment, you will have regular blood tests. This is because your medicine may cause a decrease in the number of blood cells that help fight infections (white blood cells) and the number of cells that help stop bleeding (platelets).
Your doctor will ask you to have a blood test:
- before treatment;
- every week during the first 8 weeks of treatment;
- at least once a month while you are taking Pomalidomide Teva.
Your doctor may adjust the dose of Pomalidomide Teva or stop treatment, depending on the results of these tests. Your doctor may also adjust the dose or stop this medicine due to your overall health.
Children and adolescents
Pomalidomide Teva is not recommended for use in children and adolescents under 18 years of age.
Other medicines and Pomalidomide Teva
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines. This is because Pomalidomide Teva may affect the way other medicines work. Additionally, some medicines may affect the way Pomalidomide Teva works.
In particular, tell your doctor, pharmacist, or nurse before taking Pomalidomide Teva if you are taking any of the following medicines:
- certain antifungals such as ketoconazole;
- certain antibiotics (e.g., ciprofloxacin, enoxacin);
- certain antidepressants such as fluvoxamine.
Driving and using machines
Some people experience fatigue, fainting, confusion, or decreased alertness while taking Pomalidomide Teva. If this happens to you, do not drive or use tools or machinery.
Pomalidomide Teva contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per capsule; this is essentially "sodium-free".
Pomalidomide Teva contains lactose
If your doctor has told you that you have an intolerance to some sugars, talk to them before taking this medicine.
Pomalidomide Teva contains brilliant blue FCF (E133)
This medicine contains the colorant brilliant blue FCF (E133), which may cause allergic reactions.
3. How to take Pomalidomida Teva
Pomalidomida Teva should be administered by a doctor with experience in the treatment of multiple myeloma.
Follow the medication administration instructions exactly as indicated by your doctor. In case of doubt, consult your doctor, pharmacist, or nurse.
When to take Pomalidomida Teva with other medications
Pomalidomida Teva in combination with bortezomib and dexamethasone
- Consult the package insert that comes with bortezomib and dexamethasone for additional information on their use and effects.
- Pomalidomida Teva, bortezomib, and dexamethasone are taken in treatment cycles. Each cycle lasts 21 days (3 weeks).
- Observe the following chart to see what you should take each day of the 3-week cycle:
- Each day, observe the chart and identify the correct day to see which medications you should take.
- Some days you will take all 3 medications, other days only 1 or 2 medications, and other days none of them.
POM:Pomalidomida Teva; BOR:bortezomib; DEX:dexamethasone
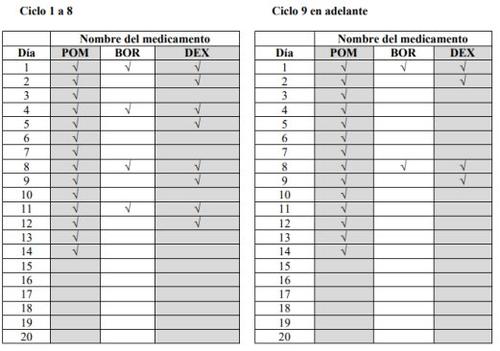
- After completing each 3-week cycle, start a new one.
Pomalidomida Teva alone with dexamethasone
- Consult the package insert that comes with dexamethasone for additional information on its use and effects.
- Pomalidomida Teva and dexamethasone are taken in treatment cycles. Each cycle lasts 28 days (4 weeks).
- Observe the following chart to see what you should take each day of the 4-week cycle:
- Each day, observe the chart and identify the correct day to see which medications you should take.
- Some days you will take both medications, other days only 1 medication, and other days none of them.
POM:Pomalidomida Teva; DEX:dexamethasone
Medication name | ||
Day | POM | DEX |
1 | √ | √ |
2 | √ | |
3 | √ | |
4 | √ | |
5 | √ | |
6 | √ | |
7 | √ | |
8 | √ | √ |
9 | √ | |
10 | √ | |
11 | √ | |
12 | √ | |
13 | √ | |
14 | √ | |
15 | √ | √ |
16 | √ | |
17 | √ | |
18 | √ | |
19 | √ | |
20 | √ | |
21 | √ | |
22 | √ | |
23 | ||
24 | ||
25 | ||
26 | ||
27 | ||
28 |
- After completing each 4-week cycle, start a new one.
How much Pomalidomida Teva to take with other medications
Pomalidomida Teva with bortezomib and dexamethasone
- The recommended initial dose of Pomalidomida Teva is 4 mg per day.
- The recommended initial dose of bortezomib will be calculated by your doctor based on your height and weight (1.3 mg/m2 of body surface area).
- The recommended initial dose of dexamethasone is 20 mg per day. However, if you are over 75 years of age, the recommended initial dose is 10 mg per day.
Pomalidomida Teva alone with dexamethasone
- The recommended dose of Pomalidomida Teva is 4 mg once a day.
- The recommended initial dose of dexamethasone is 40 mg per day. However, if you are over 75 years of age, the recommended initial dose is 20 mg per day.
Your doctor may need to reduce the dose of Pomalidomida Teva, bortezomib, or dexamethasone, or interrupt one or more of these medications based on the results of your blood tests and your overall condition, if you are taking other medications (e.g., ciprofloxacin, enoxacin, and fluvoxamine), and if you experience adverse effects (especially skin rash or swelling) as a result of treatment.
If you have liver or kidney problems, your doctor will closely monitor your condition while you are taking this medication.
How to take Pomalidomida Teva
- Do not break, open, or chew the capsules. If the powder from a broken capsule comes into contact with the skin, wash the skin immediately and thoroughly with water and soap.
- Healthcare professionals, caregivers, and family members should wear disposable gloves when handling the blister or capsule. Afterwards, they should carefully remove the gloves to avoid skin exposure, place them in a sealable polyethylene plastic bag, and dispose of them according to local requirements. Then, they should wash their hands well with water and soap. Pregnant women or those who suspect they may be pregnant should not handle the blister or capsule.
- Swallow the capsules whole, preferably with water.
- You can take the capsules with or without food.
- You should take the capsules at approximately the same time every day.
To remove the capsule from the blister, press only one end of the capsule so that it comes out through the foil. Do not press in the center of the capsule as it may break.
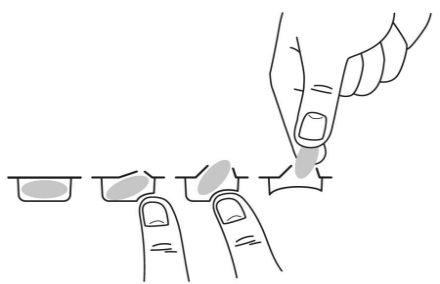
Your doctor will advise you on how and when to take Pomalidomida Teva if you have kidney problems and are receiving dialysis treatment.
Duration of treatment with Pomalidomida Teva
You should continue the treatment cycles until your doctor tells you to stop the treatment.
If you take more Pomalidomida Teva than you should
If you take more Pomalidomida Teva than you should, inform your doctor or go to the hospital immediately. Bring the medication package with you.
If you forget to take Pomalidomida Teva
If you forget to take Pomalidomida Teva on the day you should, take the next capsule the next day at the usual time. Do not take more capsules to make up for the missed dose of Pomalidomida Teva the previous day.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Severe side effects
If you experience any of the following severe side effects, stop treatment with Pomalidomida Teva and go to a doctor immediately, as you may need urgent medical treatment:
- Fever, chills, sore throat, cough, mouth ulcers, or any other sign of infection (due to a decrease in white blood cells that fight infection).
- Bleeding or bruising without apparent cause, including nosebleeds and intestinal or stomach bleeding (due to the effects on blood cells called "platelets").
- Rapid breathing, rapid heartbeat, fever, and chills, decreased ability to urinate, nausea, and vomiting, confusion, loss of consciousness (due to a blood infection called sepsis or septic shock).
- Severe diarrhea, persistent or bloody, possibly accompanied by stomach pain or fever, caused by the bacteria Clostridium difficile.
- Chest pain or leg swelling, especially in the lower leg or calf (caused by blood clots).
- Difficulty breathing (due to a severe chest infection, lung inflammation, heart failure, or blood clots).
- Swelling of the face, lips, tongue, and throat, which can cause difficulty breathing (due to severe allergic reactions called angioedema and anaphylactic reaction).
- Certain types of skin cancer (squamous cell carcinoma and basal cell carcinoma), which can cause changes in the appearance of the skin or lumps on the skin. If you notice changes in the appearance of your skin while taking Pomalidomida Teva, inform your doctor as soon as possible.
- Recurrence of hepatitis B virus infection, which can cause yellowing of the skin and eyes, dark urine, abdominal pain on the right side, fever, nausea, or discomfort. Inform your doctor immediately if you notice any of these symptoms.
- Widespread rash, high body temperature, swollen lymph nodes, and effects on other organs of the body (drug reaction with eosinophilia and systemic symptoms, also known as DRESS or drug hypersensitivity syndrome, toxic epidermal necrolysis, or Stevens-Johnson syndrome). Stop taking pomalidomide if you experience these symptoms and contact your doctor or go to the doctor immediately. See also section 2.
If you experience any of the following severe side effects, stop treatment with Pomalidomida Teva and go to a doctor immediately, as you may need urgent medical treatment.
Other side effects
Very common(may affect more than 1 in 10 people):
- Difficulty breathing (dyspnea).
- Lung infection (pneumonia and bronchitis).
- Infections in the nose, sinuses, and throat caused by bacteria or viruses.
- Flu-like symptoms (influenza).
- Low red blood cell count, which can cause anemia that leads to fatigue and weakness.
- Low potassium levels in the blood (hypokalemia), which can cause weakness, cramps, and muscle pain, palpitations, tingling or numbness, shortness of breath, and mood changes.
- High blood sugar levels.
- Fast and irregular heartbeat (atrial fibrillation).
- Lack of appetite.
- Constipation, diarrhea, or nausea.
- Vomiting.
- Abdominal pain.
- Lack of energy.
- Difficulty falling or staying asleep.
- Dizziness, tremors.
- Muscle spasms, muscle weakness.
- Bone pain, back pain.
- Numbness, tingling, or burning sensation in the skin, pain in hands or feet (peripheral sensory neuropathy).
- Generalized swelling, including swelling of arms and legs.
- Skin rashes.
- Urinary tract infection, which can cause a burning sensation when urinating or the need to urinate more frequently.
Common(may affect up to 1 in 10 people):
- Falls.
- Bleeding inside the skull.
- Decreased ability to move or feel (sensitivity) in hands, feet, and legs due to nerve damage (peripheral sensorimotor neuropathy).
- Numbness, itching, or tingling in the skin (paresthesia).
- Feeling of dizziness, which makes it difficult to stand and move normally.
- Swelling caused by fluid retention.
- Hives (urticaria).
- Itching of the skin.
- Herpes zoster.
- Heart attack (chest pain that spreads to the arms, neck, and jaw, feeling of sweating and difficulty breathing, feeling of nausea or vomiting).
- Chest pain, chest infection.
- Increased blood pressure.
- A decrease in the number of red and white blood cells and platelets at the same time (pancytopenia), which will make you more prone to bleeding and bruising. You may feel tired and weak, as well as have difficulty breathing. You will also be more susceptible to infections.
- A decrease in the number of lymphocytes (a type of white blood cell) often caused by an infection (lymphopenia).
- Low magnesium levels in the blood (hypomagnesemia), which can cause fatigue, general weakness, muscle cramps, and irritability, and can cause low calcium levels in the blood (hypocalcemia), leading to numbness or tingling in hands, feet, or lips, muscle cramps, muscle weakness, dizziness, and confusion.
- Low phosphate levels in the blood (hypophosphatemia), which can cause muscle weakness, irritability, or confusion.
- High calcium levels in the blood (hypercalcemia), which can slow down reflexes and cause weakness of skeletal muscles.
- High potassium levels in the blood, which can cause an abnormal heart rhythm.
- Low sodium levels in the blood, which can cause fatigue and confusion, muscle contractions, seizures (epileptic convulsions), or coma.
- High uric acid levels in the blood, which can cause a type of arthritis called gout.
- Low blood pressure, which can cause dizziness or fainting.
- Pain or dryness in the mouth.
- Changes in the taste of things.
- Swollen abdomen.
- Confusion.
- Feeling depressed (depressive mood).
- Loss of consciousness, fainting.
- Clouding of the eye (cataract).
- Kidney damage.
- Inability to urinate.
- Abnormal results in liver function tests.
- Pelvic pain.
- Weight loss.
Uncommon(may affect up to 1 in 100 people):
- Stroke.
- Liver inflammation (hepatitis) that can cause itching of the skin, yellowing of the skin and the white part of the eyes (jaundice), light-colored stools, dark urine, and abdominal pain.
- The breakdown of tumor cells results in the release of toxic compounds into the bloodstream (tumor lysis syndrome). It can lead to kidney problems.
- Underactive thyroid gland, which can cause symptoms such as fatigue, lethargy, muscle weakness, slow heart rate, and weight gain.
Frequency not known(cannot be estimated from the available data):
- Rejection of solid organ transplants (such as heart or liver).
Reporting side effects
If you experience any side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Pomalidomida Teva
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the blister and carton after EXP. The expiration date is the last day of the month indicated.
This medication does not require special storage conditions.
Do not use Pomalidomida Teva if you notice visible signs of deterioration or signs of tampering with the medication.
Medications should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Packaging Contents and Additional Information
Composition of Pomalidomida Teva
- The active ingredient is pomalidomide
- The other components are lactose monohydrate, crospovidone, povidone, sodium lauryl sulfate, and sodium stearyl fumarate
Pomalidomida Teva 1 mg hard capsules
- Each capsule contains 1 mg of pomalidomide
- The capsule shell contains: brilliant blue FCF (E133), titanium dioxide (E171), gelatin, and yellow iron oxide (E172)
- The printing ink contains: shellac (E904), propylene glycol (E1520), concentrated ammonia solution (E527), black iron oxide (E172), and potassium hydroxide (E525)
Pomalidomida Teva 2 mg hard capsules
- Each capsule contains 2 mg of pomalidomide
- The capsule shell contains: brilliant blue FCF (E133), titanium dioxide (E171), gelatin, yellow iron oxide (E172), and red iron oxide (E172)
- The printing ink contains: shellac (E904), propylene glycol (E1520), concentrated ammonia solution (E527), black iron oxide (E172), and potassium hydroxide (E525)
Pomalidomida Teva 3 mg hard capsules
- Each capsule contains 3 mg of pomalidomide
- The capsule shell contains: brilliant blue FCF (E133), titanium dioxide (E171), gelatin, and yellow iron oxide (E172)
- The printing ink contains: shellac (E904), propylene glycol (E1520), concentrated ammonia solution (E527), black iron oxide (E172), and potassium hydroxide (E525)
Pomalidomida Teva 4 mg hard capsules
- Each capsule contains 4 mg of pomalidomide
- The capsule shell contains: brilliant blue FCF (E133), titanium dioxide (E171), and gelatin
- The printing ink contains: shellac (E904), propylene glycol (E1520), concentrated ammonia solution (E527), black iron oxide (E172), and potassium hydroxide (E525)
Product Appearance and Packaging Contents
Pomalidomida Teva 1 mg hard capsules: hard gelatin capsule of approximately 14 mm with an opaque blue cap and an opaque yellow body with the inscription “T” on the cap and “1” on the body.
Pomalidomida Teva 2 mg hard capsules: hard gelatin capsule of approximately 18 mm with an opaque blue cap and an opaque orange body with the inscription “T” on the cap and “2” on the body.
Pomalidomida Teva 3 mg hard capsules: hard gelatin capsule of approximately 18 mm with an opaque blue cap and an opaque green body with the inscription “T” on the cap and “3” on the body.
Pomalidomida Teva 4 mg hard capsules: hard gelatin capsule of approximately 18 mm with an opaque blue cap and an opaque light blue body with the inscription “T” on the cap and “4” on the body.
Each pack contains 14, 14 x 1, 21, 21 x 1, 63, and 63 x 1 capsules. Not all pack sizes may be marketed.
Marketing Authorisation Holder
TEVA GmbH
Graf-Arco-Str. 3
89079 Ulm
Germany
Manufacturer
Balkanpharma-Dupnitsa AD
3 Samokovsko Shosse Str.
Dupnitsa 2600, Bulgaria
Merckle GmbH
Graf-Arco-Str. 3
89079 Ulm, Germany
Actavis Group PTC ehf.
Dalshraun 1
IS-220 Hafnarfjordur, Iceland
You can request more information about this medicinal product by contacting the local representative of the marketing authorisation holder:
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A. /AG Tel/Tél: +32 3 820 73 73 | Lietuva UAB Teva Baltics Tel: +370 5 266 02 03 |
| Luxembourg/Luxemburg ratiopharm GmbH Tél: +49 731 402 02 |
Ceská republika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251 007 111 | Magyarország Teva Gyógyszergyár Zrt. Tel: (+36) 1 288 6400 |
Danmark Teva Denmark A/S Tlf.: +45 44 98 55 11 | Malta Teva Pharmaceuticals Ireland Tel: +44 (0) 207 540 7117 |
Deutschland ratiopharm GmbH +49 (0) 731 402 02 | Nederland Teva Nederland B.V. Tel: +31 800 0228 400 |
Eesti UAB Teva Baltics Eesti filiaal Tel.: +372 6610801 | Norge Teva Norway AS Tlf: +47 66 77 55 90 |
| Österreich Ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
España Teva Pharma, S.L.U. Tel.: + 34 91 387 32 80 | Polska Teva Pharmaceuticals Polska Sp. z o.o. Tel.: +48 22 345 93 00 |
France Teva Santé Tél: +33 1 55 91 78 00 | Portugal Teva Pharma - Produtos Farmacêuticos, Lda Tel: +351 21 476 75 50 |
Hrvatska Pliva Hrvatska d.o.o Tel: + 385 1 37 20 000 | România Teva Pharmaceuticals S.R.L Tel: +40 21 230 65 24 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 (0) 207 540 7117 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 1 58 90 390 |
Ísland Teva Pharma Iceland ehf. Sími: + 354 550 3300 | Slovenská republika TEVA Pharmaceuticals Slovakia s.r.o Telephone: +421257267911 |
Italia Teva Italia S.r.l Tel:. +39 028917981 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 20 180 5900 |
| Sverige Teva Sweden AB Tel: +46 (0)42 12 11 00 |
Latvija UAB Teva Baltics filiale Latvija Tel: +371 67 323 666 | United Kingdom (Northern Ireland) United Kingdom (Northern Ireland) Teva Pharmaceuticals Ireland Ireland Tel: +44 (0) 207 540 7117 |
Date of the Last Revision of this Leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to POMALIDOMIDE TEVA 4 mg HARD CAPSULESDosage form: CAPSULE, 2 mgActive substance: pomalidomideManufacturer: Bristol-Myers Squibb Pharma EeigPrescription requiredDosage form: CAPSULE, 3mgActive substance: pomalidomideManufacturer: Bristol-Myers Squibb Pharma EeigPrescription requiredDosage form: CAPSULE, 4mgActive substance: pomalidomideManufacturer: Bristol-Myers Squibb Pharma EeigPrescription required
Online doctors for POMALIDOMIDE TEVA 4 mg HARD CAPSULES
Discuss questions about POMALIDOMIDE TEVA 4 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions









