
POMALIDOMIDE GRINDEKS 4 mg HARD CAPSULES

How to use POMALIDOMIDE GRINDEKS 4 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Pomalidomida Grindeks 1mg hard capsules EFG
Pomalidomida Grindeks 2mg hard capsules EFG
Pomalidomida Grindeks 3mg hard capsules EFG
Pomalidomida Grindeks 4mg hard capsules EFG

Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Pomalidomida Grindeks and what is it used for
- What you need to know before you take Pomalidomida Grindeks
- How to take Pomalidomida Grindeks
- Possible side effects
- Storage of Pomalidomida Grindeks
- Contents of the pack and other information
1. What is Pomalidomida Grindeks and what is it used for
What is Pomalidomida Grindeks
Pomalidomida Grindeks contains the active substance pomalidomide. This medicine is related to thalidomide and belongs to a group of medicines that affect the immune system (the body's natural defenses).
What is Pomalidomida Grindeks used for
Pomalidomida Grindeks is used to treat adults with a type of cancer called multiple myeloma.
Pomalidomide is used with:
- Other two medicines, called bortezomib(a type of chemotherapy medicine) and dexamethasone(an anti-inflammatory medicine) in people who have received at least one previous treatment, including lenalidomide.
U
- Another medicine, called dexamethasone, in people whose myeloma has worsened despite receiving at least two other treatments, including lenalidomide and bortezomib.
What is multiple myeloma
Multiple myeloma is a type of cancer that affects a specific type of white blood cell (called plasma cells). These cells grow out of control and accumulate in the bone marrow. As a result, the bones and kidneys are damaged.
Multiple myeloma usually has no cure. However, treatment can reduce the signs and symptoms of the disease or make them disappear for a period of time. When this happens, it is called a response.
How Pomalidomida Grindeks works
Pomalidomide works in different ways:
- interrupting the development of myeloma cells
- stimulating the immune system to attack cancer cells
- interrupting the formation of blood vessels that feed cancer cells
Benefit of using pomalidomide with bortezomib and dexamethasone
When pomalidomide is used with bortezomib and dexamethasone in people who have received at least one other treatment, it can delay the worsening of multiple myeloma.
Generally, the combination of pomalidomide with bortezomib and dexamethasone prevents multiple myeloma from coming back for up to 11 months, compared to 7 months in patients who only use bortezomib and dexamethasone.
Benefit of using pomalidomide with dexamethasone
When pomalidomide is used with dexamethasone in people who have received at least two other treatments, it can delay the worsening of multiple myeloma.
Generally, the combination of pomalidomide with dexamethasone prevents multiple myeloma from coming back for up to 4 months, compared to 2 months in patients who only use dexamethasone.
2. What you need to know before you take Pomalidomida Grindeks
Do not take Pomalidomida Grindeks:
- if you are pregnant, think you may be pregnant or plan to become pregnant, as pomalidomide is expected to be harmful to the fetus. (Men and women taking this medicine should read the section on Pregnancy, contraception and breastfeeding: information for men and womenlater in this leaflet).
- if you can become pregnant, unless you use effective contraception (see the section on Pregnancy, contraception and breastfeeding: information for men and women). If you can become pregnant, your doctor will confirm that you have taken the necessary measures to prevent pregnancy before prescribing the medicine.
- if you are allergic to pomalidomide or any of the other ingredients of this medicine (listed in section 6). If you think you may be allergic, consult your doctor for advice.
If you are not sure if any of these situations apply to you, consult your doctor, pharmacist or nurse before taking pomalidomide.
Warnings and precautions
Consult your doctor, pharmacist or nurse before starting treatment with Pomalidomida Grindeks if:
- You have had blood clots in the past. During treatment with pomalidomide, you have a higher risk of developing blood clots in your veins and arteries. Your doctor may recommend that you take additional treatments (e.g. warfarin) or reduce the dose of pomalidomide to reduce the likelihood of developing blood clots.
- You have ever had an allergic reaction, such as a rash, itching, swelling, dizziness or difficulty breathing while taking medicines related to thalidomideor lenalidomide.
- You have had a heart attack, have heart failure, have difficulty breathing or if you smoke, have high blood pressure or high cholesterol levels.
- You have a high tumor burden in your body, including the bone marrow. This could lead to a condition where the tumors break down and produce unusual levels of chemicals in the blood, which can cause kidney failure. You may also experience irregular heartbeats. This condition is called tumor lysis syndrome.
- You suffer or have suffered from neuropathy (nerve damage that causes tingling or pain in the feet or hands).
- You have or have had a hepatitis B virus infection. Treatment with pomalidomide may reactivate the hepatitis B virus in patients who are carriers of the virus, leading to the infection coming back (recurrence). Your doctor must check if you have ever had a hepatitis B virus infection.
- You experience or have experienced in the past a combination of any of the following symptoms: rash on the face or generalized, skin redness, high fever, flu-like symptoms, swollen lymph nodes (symptoms of a severe skin reaction called drug reaction with eosinophilia and systemic symptoms[DRESS] or hypersensitivity syndrome to drugs, toxic epidermal necrolysis[TEN] or Stevens-Johnson syndrome[SSJ]. See also section 4 “Possible side effects”.
It is important to note that patients with multiple myeloma treated with pomalidomide may develop other types of cancer, so your doctor must carefully evaluate the benefits and risks when prescribing this medicine.
At any time during or after treatment, tell your doctor or nurse immediately if you: have blurred vision, loss of vision or double vision, difficulty speaking, weakness in an arm or leg, a change in the way you walk or balance problems, persistent numbness, decreased sensitivity or loss of sensitivity, memory loss or confusion. These may be symptoms of a serious and potentially life-threatening brain disease called progressive multifocal leukoencephalopathy(PML). If you had any of these symptoms before starting treatment with pomalidomide, tell your doctor if you notice any change in these symptoms.
At the end of treatment, you must return all unused capsules to the pharmacist.
Pregnancy, contraception and breastfeeding: information for men and women
You must follow the instructions in the Pomalidomide Pregnancy Prevention Programme. Men and women taking pomalidomide must not father a child or become pregnant. The reason is that pomalidomide is expected to be harmful to the fetus. You and your partner must use effective contraception while taking this medicine.
Women
Do not take pomalidomide if you are pregnant, think you may be pregnant or plan to become pregnant. The reason is that this medicine is expected to be harmful to the fetus. Before starting treatment, you must tell your doctor if there is a possibility that you may become pregnant, even if you think this is unlikely.
If you can become pregnant:
- You must use effective contraception from at least 4 weeks before starting treatment, during treatment and for at least 4 weeks after stopping treatment. Your doctor will advise you on the most suitable contraceptive methods for you.
- Each time your doctor prescribes a prescription, they will ensure that you have understood the necessary measures to prevent pregnancy.
- Your doctor will schedule pregnancy tests before treatment, at least every 4 weeks during treatment and at least 4 weeks after stopping treatment.
If, despite the preventive measures, you become pregnant:
- You must stop treatment immediately and inform your doctor immediately.
Breastfeeding
It is not known whether pomalidomide is excreted in breast milk. Inform your doctor if you are breastfeeding or plan to breastfeed. Your doctor will advise you whether you can continue breastfeeding or should stop.
Men
Pomalidomide passes into human semen.
- If your partner is pregnant or can become pregnant, you must use condoms during treatment and for 7 days after stopping treatment.
- If your partner becomes pregnant while you are taking pomalidomide, inform your doctor immediately. Your partner must also inform their doctor immediately.
You must not donate sperm or semen during treatment and for 7 days after stopping treatment.
Blood donation and blood tests
You must not donate blood during treatment and for 7 days after stopping treatment.
Before starting treatment with pomalidomide and during treatment, you will have regular blood tests. This is because the medicine can cause a decrease in the number of blood cells that help fight infections (white blood cells) and the number of cells that help stop bleeding (platelets).
Your doctor must request that you have a blood test:
- before treatment
- every week for the first 8 weeks of treatment
- at least once a month while you are taking pomalidomide
Your doctor may adjust the dose of pomalidomide or stop treatment, depending on the results of these tests. The doctor may also change the dose or stop this medicine due to your general state of health.
Children and adolescents
Pomalidomide is not recommended for use in children and adolescents under 18 years of age.
Other medicines and Pomalidomida Grindeks
Tell your doctor, pharmacist or nurse if you are taking, have recently taken or might take any other medicines. This is because pomalidomide can affect the way other medicines work. Other medicines can also affect the way pomalidomide works.
In particular, tell your doctor, pharmacist or nurse before taking pomalidomide if you are taking any of the following medicines:
- certain antifungals, such as ketoconazole
- certain antibiotics (e.g. ciprofloxacin, enoxacin)
- certain antidepressants, such as fluvoxamine
Driving and using machines
Some people experience fatigue, dizziness, fainting, confusion or decreased alertness while taking pomalidomide. If you experience these effects, avoid driving or using tools or machines.
Pomalidomida Grindeks contains sodium.
This medicine contains less than 1 mmol of sodium (23 mg) per capsule; this is essentially ‘sodium-free’.
Pomalidomida Grindeks contains azo colorants
The capsules contain the azo colorants brilliant black PN (all strengths), carmoisine, azorubine (all strengths) and orange yellow FCF (only the 2 mg capsules). These colorants may cause allergic reactions.
3. How to take Pomalidomida Grindeks
Pomalidomida should be administered by a doctor with experience in the treatment of multiple myeloma.
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor, pharmacist, or nurse again.
When to take Pomalidomida Grindeks with other medications
Pomalidomida with bortezomib and dexamethasone
- Consult the leaflet that comes with bortezomib and dexamethasone for additional information on their use and effects.
- Pomalidomida, bortezomib, and dexamethasone are taken in "treatment cycles". Each cycle lasts 21 days (3 weeks).
- Observe the following chart to see what you should take each day of the 3-week cycle.
- Each day, consult the chart and identify the correct day to see which medications you should take.
- Some days you will take all 3 medications; other days, only 1 or 2 medications; and other days, none of them.
PMD:Pomalidomida; BOR: Bortezomib; DEX: Dexamethasone
Cycle1 to 8 and subsequentCycle9 and subsequent
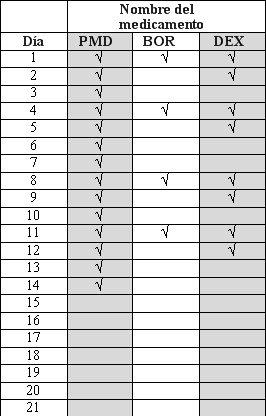
After completing each 3-week cycle, start a new one.
Pomalidomida alone with dexamethasone
- Consult the leaflet that comes with dexamethasone for additional information on its use and effects.
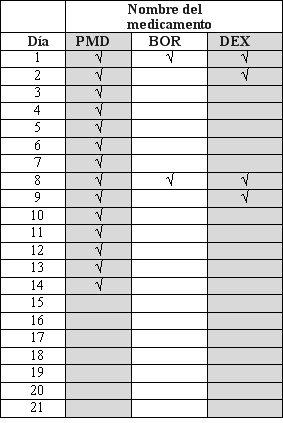
- Pomalidomida and dexamethasone are taken in "treatment cycles". Each cycle lasts 28 days (4 weeks).
- Observe the following chart to see what you should take each day of the 4-week cycle:
- Each day, consult the chart and identify the correct day to see which medications you should take.
- Some days you will take both medications; other days, only 1; and other days, none of them.
PMD:Pomalidomida; BOR: Dexamethasone
Medication Name | ||
Day | PMD | DEX |
1 | √ | √ |
2 | √ | |
3 | √ | |
4 | √ | |
5 | √ | |
6 | √ | |
7 | √ | |
8 | √ | √ |
9 | √ | |
10 | √ | |
11 | √ | |
12 | √ | |
13 | √ | |
14 | √ | |
15 | √ | √ |
16 | √ | |
17 | √ | |
18 | √ | |
19 | √ | |
20 | √ | |
21 | √ | |
22 | √ | |
23 | ||
24 | ||
25 | ||
26 | ||
27 | ||
28 |
After completing each 4-week cycle, start a new one.
How much pomalidomida to take with other medications
Pomalidomida with bortezomib and dexamethasone
- The recommended initial dose of pomalidomida is 4 mg per day.
- The recommended initial dose of bortezomib will be calculated by your doctor based on your height and weight (1.3 mg/m2 of body surface area).
- The recommended initial dose of dexamethasone is 20 mg per day. However, if you are over 75 years old, the recommended initial dose is 10 mg per day.
Pomalidomida alone with dexamethasone
- The recommended dose of pomalidomida is 4 mg per day.
- The recommended starting dose of dexamethasone is 40 mg per day. However, if you are over 75 years old, the recommended initial dose is 20 mg per day.
It is possible that your doctor may need to reduce the dose of pomalidomida, bortezomib, or dexamethasone or interrupt one or more of these medications based on the results obtained in blood tests and your general condition, if you are taking other medications (e.g., ciprofloxacin, enoxacin, and fluvoxamine) and if you experience adverse effects (especially rash or swelling) as a consequence of treatment.
If you have liver or kidney problems, your doctor will carefully monitor your condition while you are receiving this medication.
How to take Pomalidomida Grindeks
- Do not chew, break, or open the capsules. If the powder from a broken pomalidomida capsule comes into contact with the skin, wash the skin immediately and thoroughly with water and soap.
- Healthcare professionals, caregivers, and family members should wear disposable gloves when handling the blister or capsule. Afterwards, they should carefully remove the gloves to avoid skin exposure, place them in a sealable polyethylene plastic bag, and dispose of them according to local requirements. Then, they should wash their hands thoroughly with water and soap. Pregnant women or those who suspect they may be pregnant should not handle the blister or capsule.
- Swallow the capsules whole, preferably with water.
- You can take the capsules with or without food.
- Take pomalidomida approximately at the same time each day.
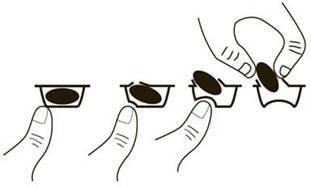
To remove the capsule from the blister, press only one end of the capsule so that it comes out through the foil. Do not press the center of the capsule, as it may break.
Your doctor will advise you on how and when to take pomalidomida if you have kidney problems and are receiving dialysis treatment.
Duration of treatment with Pomalidomida Grindeks
You should continue the treatment cycles until your doctor tells you to stop the treatment.
If you take more Pomalidomida Grindeks than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, indicating the medication and the amount ingested.
If you forget to take Pomalidomida Grindeks
If you forgot to take pomalidomida on the day you should, take the next capsule the next day at the usual time. Do not take a double dose to make up for the missed doses.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not all people experience them.
Severe side effects
If you experience any of the following severe side effects, stop treatment with pomalidomida and go to a doctor immediately because you may need urgent medical treatment:
- Fever, chills, sore throat, cough, mouth ulcers, or any other sign of infection (due to a decrease in white blood cells that fight infections).
- Bleeding or bruising without apparent cause, including nosebleeds and intestinal or stomach bleeding (due to effects on blood cells called platelets).
- Rapid breathing, rapid pulse, fever, and chills, decreased ability to urinate, nausea, and vomiting, confusion, loss of consciousness (due to a blood infection called sepsis or septic shock).
- Severe diarrhea, persistent or bloody (possibly accompanied by stomach pain or fever) caused by the bacteria Clostridium difficile.
- Chest pain or leg swelling (caused by blood clots).
- Shortness of breath (due to a severe chest infection, pneumonia, heart failure, or blood clots).
- Swelling of the face, lips, tongue, and throat, which can cause difficulty breathing (due to severe allergic reactions called angioedema and anaphylactic reaction).
- Certain types of skin cancer (squamous cell carcinoma and basal cell carcinoma), which can cause changes in the appearance of the skin or lumps on the skin. If you notice changes in the appearance of your skin while taking pomalidomida, inform your doctor as soon as possible.
- Recurrence of hepatitis B virus infection, which can cause yellowing of the skin and eyes, dark urine, abdominal pain on the right side, fever, nausea, or discomfort. Inform your doctor immediately if you experience any of these symptoms.
- Widespread rash, high body temperature, swollen lymph nodes, and involvement of other organs (drug reaction with eosinophilia and systemic symptoms, also known as "DRESS" or "drug-induced hypersensitivity syndrome", toxic epidermal necrolysis, or Stevens-Johnson syndrome). If you present these symptoms, stop taking pomalidomida and contact your doctor or seek immediate medical attention. See also section 2.
If you experience any of the following severe side effects, stop treatment with pomalidomida and go to a doctor immediately because you may need urgent medical treatment.
Other side effects
Very common(may affect more than 1 in 10 people):
- Shortness of breath (dyspnea).
- Lung infection (pneumonia and bronchitis).
- Infections in the nose, sinuses, and throat caused by bacteria or viruses.
- Flu-like symptoms.
- Low red blood cell count, which can cause anemia that leads to fatigue and weakness.
- Low potassium levels in the blood (hypokalemia), which can cause weakness, cramps, and muscle pain, palpitations, tingling or numbness, shortness of breath, and mood changes.
- High blood sugar levels.
- Fast and irregular heartbeat (atrial fibrillation).
- Lack of appetite.
- Constipation, diarrhea, or nausea.
- Nausea and vomiting.
- Abdominal pain.
- Lack of energy.
- Difficulty staying asleep or falling asleep.
- Dizziness, tremors.
- Muscle spasms, muscle weakness.
- Bone pain, back pain.
- Numbness, tingling, or burning sensation in the skin, pain in the hands or feet (peripheral sensory neuropathy).
- Generalized swelling, including swelling of the arms and legs.
- Skin rash.
- Urinary tract infection, which can cause a burning sensation when urinating or the need to urinate more frequently.
Common(may affect up to 1 in 10 people):
- Falls.
- Bleeding inside the skull.
- Decreased ability to move or feel (sensitivity) in the hands, feet, and legs due to nerve damage (peripheral sensorimotor neuropathy).
- Numbness, itching, and tingling in the skin (paresthesia).
- Feeling of dizziness, which makes it difficult to stand and move normally.
- Swelling caused by fluid retention.
- Hives (urticaria).
- Itching of the skin.
- Shingles.
- Heart attack (chest pain that spreads to the arms, neck, and jaw, feeling of sweating and difficulty breathing, feeling of nausea or vomiting).
- Chest pain, chest infection.
- High blood pressure.
- A decrease in the number of red and white blood cells and platelets at the same time (pancytopenia) that will make you more prone to bleeding and bruising. You may feel tired and weak, as well as have difficulty breathing, and you will also be more susceptible to infections.
- A decrease in the number of lymphocytes (a type of white blood cell) often caused by an infection (lymphopenia).
- Low magnesium levels in the blood (hypomagnesemia), which can cause fatigue, general weakness, muscle cramps, and irritability, and can also cause low calcium levels in the blood (hypocalcemia), leading to numbness or tingling in the hands, feet, or lips, muscle cramps, muscle weakness, dizziness, and confusion.
- Low phosphate levels in the blood (hypophosphatemia), which can cause muscle weakness, irritability, or confusion.
- High calcium levels in the blood (hypercalcemia), which can slow down reflexes and cause weakness in skeletal muscles.
- High potassium levels in the blood, which can cause an abnormal heart rhythm.
- Low sodium levels in the blood, which can cause fatigue and confusion, muscle contractions, seizures (epileptic convulsions), or coma.
- High uric acid levels in the blood, which can cause a type of arthritis called gout.
- Low blood pressure, which can cause dizziness or fainting.
- Pain or dryness in the mouth.
- Changes in the taste of things.
- Swollen abdomen.
- Feeling of confusion.
- Feeling depressed (depressive mood).
- Loss of consciousness, fainting.
- Clouding of the eye (cataract).
- Kidney damage.
- Inability to urinate.
- Abnormal results in liver function tests.
- Pelvic pain.
- Weight loss.
Uncommon(may affect up to 1 in 100 people):
- Stroke.
- Liver inflammation (hepatitis), which can cause itching of the skin, yellowing of the skin and the white part of the eyes (jaundice), light-colored stools, dark urine, and abdominal pain.
- The breakdown of cancer cells results in the release of toxic compounds into the bloodstream (tumor lysis syndrome). It can lead to kidney problems.
- Underactive thyroid gland, which can cause symptoms such as fatigue, lethargy, muscle weakness, slow heart rate, weight gain.
Frequency not known(cannot be estimated from the available data):
Rejection of solid organ transplants (such as heart or liver).
Reporting side effects
If you experience any side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Pomalidomida Grindeks
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date that appears on the blister and carton after CAD. The expiration date is the last day of the month indicated.
This medication does not require special storage conditions.
Do not use this medication if you observe deterioration or signs of improper handling of the medication.
Medications should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition of Pomalidomida Grindeks
Pomalidomida Grindeks 1 mg hard capsules EFG:
- The active substance is pomalidomide. Each capsule contains 1 mg of pomalidomide.
- The other components are pregelatinized starch, maltodextrin, crospovidone, anhydrous colloidal silica, and sodium stearyl fumarate.
- The capsule shell contains gelatin, titanium dioxide (E-171), and colorants: (yellow iron oxide (E-172), black iron oxide (E-172), black PN brilliant (E-151), patent blue V (E-131), carmoisine, azorubine (E-122), blue brilliant FCF (E-133), and white printing ink (shellac, titanium dioxide [E-171], sodium hydroxide, propylene glycol [E-1520], and povidone [E-1201]).
Pomalidomida Grindeks 2 mg hard capsules EFG:
- The active substance is pomalidomide. Each capsule contains 2 mg of pomalidomide.
- The other components are pregelatinized starch, maltodextrin, crospovidone, anhydrous colloidal silica, and sodium stearyl fumarate.
- The capsule shell contains gelatin, titanium dioxide (E-171), and colorants: (orange yellow FCF (E-110), black PN brilliant (E-151), patent blue V (E-131), carmoisine, azorubine (E-122), and white printing ink (shellac, titanium dioxide [E-171], sodium hydroxide, propylene glycol [E-1520], and povidone [E-1201]).
Pomalidomida Grindeks 3 mg hard capsules EFG:
- The active substance is pomalidomide. Each capsule contains 3 mg of pomalidomide.
- The other components are pregelatinized starch, maltodextrin, crospovidone, anhydrous colloidal silica, and sodium stearyl fumarate.
- The capsule shell contains gelatin, titanium dioxide (E-171), and colorants: (black PN brilliant (E-151), patent blue V (E-131), carmoisine, azorubine (E-122), blue brilliant FCF (E-133), erythrosine (E-127), and white printing ink (shellac, titanium dioxide [E-171], sodium hydroxide, propylene glycol [E-1520], and povidone [E-1201]).
Pomalidomida Grindeks 4 mg hard capsules EFG:
- The active substance is pomalidomide. Each capsule contains 4 mg of pomalidomide.
- The other components are pregelatinized starch, maltodextrin, crospovidone, anhydrous colloidal silica, and sodium stearyl fumarate.
- The capsule shell contains gelatin, titanium dioxide (E-171), and colorants: (blue brilliant FCF (E-133), black PN brilliant (E-151), patent blue V (E-131), carmoisine, azorubine (E-122), erythrosine (E-127), and white printing ink (shellac, titanium dioxide [E-171], sodium hydroxide, propylene glycol [E-1520], and povidone [E-1201]).
Appearance of the product and container contents
Pomalidomida Grindeks 1 mg hard capsules are hard gelatin capsules, size 4 (approximately 14 mm x 5 mm), with a light gray body with the imprint P1 in white ink and an opaque dark blue cap.
Pomalidomida Grindeks 2 mg hard capsules are hard gelatin capsules, size 3 (approximately 16 mm x 6 mm), with an opaque orange body with the imprint P2 in white ink and an opaque dark blue cap.
Pomalidomida Grindeks 3 mg hard capsules are hard gelatin capsules, size 2 (approximately 18 mm x 6 mm), with a light blue body with the imprint P3 in white ink and an opaque dark blue cap.
Pomalidomida Grindeks 4 mg hard capsules are hard gelatin capsules, size 1 (approximately 19 mm x 7 mm), with an opaque blue body with the imprint P4 in white ink and an opaque dark blue cap.
The capsules are presented in containers of 21 capsules (3 blisters per container, with 7 capsules in each blister).
Marketing authorization holder and manufacturer
AS GRINDEKS
Krustpils iela 53,
Riga, LV-1057,
Latvia
You can request more information about this medicine by contacting the local representative of the marketing authorization holder
Grindeks Kalceks España, S.L.
C/ José Abascal, 58 – 2º Dcha.
28003, Madrid, Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria | Pomalidomid Grindeks 1 mg, 2 mg, 3 mg, 4 mg Hartkapseln |
Belgium | Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg gélules |
Bulgaria | ??????????? ???????? 1 mg, 2 mg, 3 mg, 4 mg ?????? ??????? Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg hard capsules |
Czech Republic | Pomalidomide Grindeks |
Denmark | Pomalidomid Grindeks |
Estonia | Pomalidomide Grindeks |
Finland | Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg kovat kapselit |
France | POMALIDOMIDE GRINDEKS 1 mg, gélule POMALIDOMIDE GRINDEKS 2 mg, gélule POMALIDOMIDE GRINDEKS 3 mg, gélule POMALIDOMIDE GRINDEKS 4 mg, gélule |
Germany | Pomalidomid Grindeks 1 mg, 2 mg, 3 mg, 4 mg Hartkapseln |
Greece | Pomalidomide/Grindeks |
Hungary | Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg kemény kapszula |
Ireland | Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg hard capsule |
Italy | Pomalidomide Grindeks |
Latvia | Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg cietas kapsulas |
Lithuania | Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg kietosios kapsules |
Netherlands | Pomalidomide Grindeks 1 mg harde capsules Pomalidomide Grindeks 2 mg harde capsules Pomalidomide Grindeks 3 mg harde capsules Pomalidomide Grindeks 4 mg harde capsules |
Norway | Pomalidomide Grindeks |
Poland | Pomalidomide Grindeks |
Portugal | Pomalidomida Grindeks 1 mg, 2 mg, 3 mg, 4 mg cápsula |
Romania | Pomalidomida Grindeks 1 mg capsule Pomalidomida Grindeks 2 mg capsule Pomalidomida Grindeks 3 mg capsule Pomalidomida Grindeks 4 mg capsule |
Slovenia | Pomalidomid Grindeks 1 mg, 2 mg, 3 mg, 4 mg trde kapsule |
Slovakia | Pomalidomid Grindeks 1 mg tvrdé kapsuly Pomalidomid Grindeks 2 mg tvrdé kapsuly Pomalidomid Grindeks 3 mg tvrdé kapsuly Pomalidomid Grindeks 4 mg tvrdé kapsuly |
Spain | Pomalidomida Grindeks 1 mg hard capsules EFG Pomalidomida Grindeks 2 mg hard capsules EFG Pomalidomida Grindeks 3 mg hard capsules EFG Pomalidomida Grindeks 4 mg hard capsules EFG |
Sweden | Pomalidomide Grindeks 1 mg, 2 mg, 3 mg, 4 mg hårda kapslar |
Date of the last revision of this prospectus: 10/2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to POMALIDOMIDE GRINDEKS 4 mg HARD CAPSULESDosage form: CAPSULE, 2 mgActive substance: pomalidomideManufacturer: Bristol-Myers Squibb Pharma EeigPrescription requiredDosage form: CAPSULE, 3mgActive substance: pomalidomideManufacturer: Bristol-Myers Squibb Pharma EeigPrescription requiredDosage form: CAPSULE, 4mgActive substance: pomalidomideManufacturer: Bristol-Myers Squibb Pharma EeigPrescription required
Online doctors for POMALIDOMIDE GRINDEKS 4 mg HARD CAPSULES
Discuss questions about POMALIDOMIDE GRINDEKS 4 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






