
PHYSIONEAL 40 3.86% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS 38.6 mg/ml

How to use PHYSIONEAL 40 3.86% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS 38.6 mg/ml
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
PHYSIONEAL 40 Glucose 1.36% w/v/ 13.6 mg/ml, Solution for Peritoneal Dialysis
PHYSIONEAL 40 Glucose 2.27% w/v/ 22.7 mg/ml, Solution for Peritoneal Dialysis
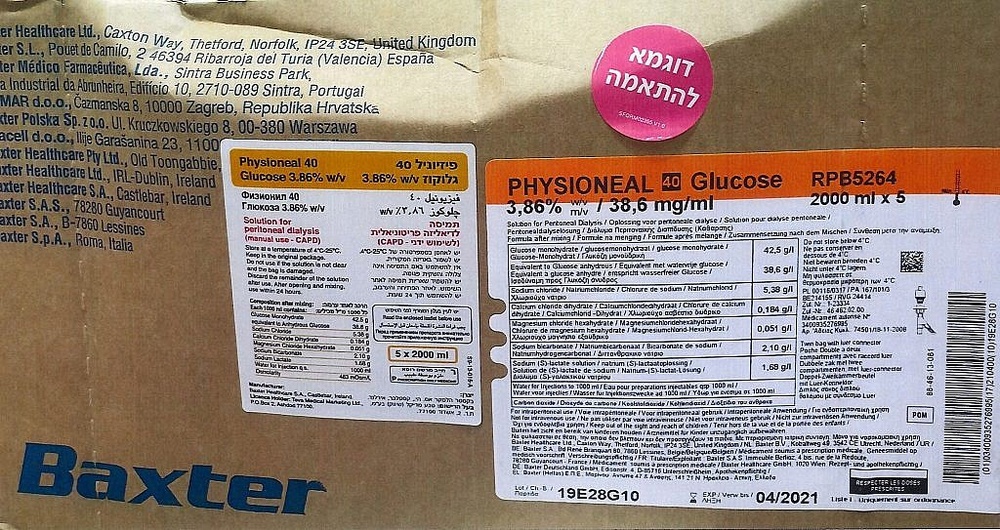
PHYSIONEAL 40 Glucose 3.86% w/v/ 38.6 mg/ml, Solution for Peritoneal Dialysis
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What PHYSIONEAL 40 is and what it is used for
- What you need to know before you use PHYSIONEAL 40
- How to use PHYSIONEAL 40
- Possible side effects
- Storage of PHYSIONEAL 40
- Contents of the pack and other information
1. What PHYSIONEAL 40 is and what it is used for
PHYSIONEAL 40 is a solution for peritoneal dialysis. It removes water and waste products from the blood. It also corrects abnormal levels of various blood components.
PHYSIONEAL 40 contains different concentrations of glucose (1.36%, 2.27%, or 3.86%). The higher the glucose concentration of the solution, the more water will be removed from the blood.
PHYSIONEAL 40 may be prescribed for you if you have:
- temporary or permanent kidney failure
- severe water retention
- severe acid-base imbalance (pH) and electrolyte disturbances in the blood
- certain types of poisoning due to medications for which there are no other available treatments.
The PHYSIONEAL 40 solution has an acidity (pH) similar to that of blood. For this reason, it may be particularly useful if you experience discomfort or pain during the administration of other more acidic peritoneal dialysis solutions.
2. What you need to know before you use PHYSIONEAL 40
Your doctor should supervise the administration of this product if it is the first time you are using it.
Do not use PHYSIONEAL 40
- if you are allergic to the active substances or to any of the other components of this medicine (listed in section 6),
- if you have any non-correctable surgical problem affecting your abdominal wall or cavity
or an uncorrectable condition that increases the risk of abdominal infections
- if you have a documented loss of peritoneal function due to severe peritoneal scarring.
Warnings and precautions
Consult your doctor before starting to use PHYSIONEAL 40.
Be particularly cautious:
- Tell your doctor: If you have serious problems affecting the integrity of the abdominal wall or cavity. For example, in case of hernia or chronic infection or inflammatory disease affecting the intestines.
- If you have an aortic graft.
- If you have severe breathing difficulties.
- If you experience abdominal pain, elevated body temperature, or notice that the drainage fluid is cloudy or contains particles. This may be a sign of peritonitis (inflamed peritoneum) or infection. You should contact your medical team urgently. Note the batch number of the peritoneal dialysis solution bags you are using and take it with the drained fluid bag to the medical team. The medical team will decide whether to interrupt treatment or initiate corrective treatment. For example, if you have an infection, your doctor may perform several tests to determine which antibiotic is most suitable for you. Your doctor may give you a broad-spectrum antibiotic effective against a wide range of
bacteria until they know what infection you have. This type of antibiotic is called a broad-spectrum antibiotic.
- If you have high levels of lactate in your blood. You have a high risk of lactic acidosis if:
- you have profoundly low blood pressure
- you have a blood infection
- you have acute kidney failure
- you have a congenital metabolic disorder
- you are taking metformin (a medication used to treat diabetes)
- you are taking medications to treat HIV, especially those called NRTIs.
- If you have diabetes and use this solution, the dose of your medications used to regulate blood sugar levels (e.g., insulin) should be reviewed regularly. Especially, the dose of your diabetes medications should be adjusted when peritoneal dialysis treatment is initiated or changed.
- If you have a corn allergy that may lead to hypersensitivity reactions, including severe allergic reactions known as anaphylaxis. Stop administration immediately and drain the solution from the peritoneal cavity.
- If you have high levels of parathyroid hormone in your blood due to your kidney condition. The low calcium levels in PHYSIONEAL 40 may worsen your
hyperparathyroidism. Your doctor will monitor your parathyroid hormone blood levels.
- You, probably in conjunction with your doctor, will keep a record of your fluid balance and body weight. Your doctor will regularly check your blood parameters. In particular, electrolytes (e.g., bicarbonate, potassium, magnesium, calcium, and phosphate), parathyroid hormone, and lipids.
- If you have high bicarbonate levels in your blood.
- Do not use more solution than prescribed by your doctor. Symptoms of excessive administration include abdominal distension, stomach heaviness, and difficulty breathing.
Your doctor will regularly check your potassium levels. If the levels drop too low, you may be given potassium chloride to compensate.
- An inadequate sequence of priming or clamping can produce air perfusion into the peritoneal cavity, which can cause abdominal pain and/or peritonitis.
- If you administer unmixed solution, you should immediately perform drainage (emptying) of the solution and use a new mixed bag.
- Due to a disorder called encapsulating peritoneal sclerosis (EPS), which is a rare and known complication of peritoneal dialysis therapy, you, probably in conjunction with your doctor, should be aware of this possible complication. EPS causes:
abdominal inflammation (belly)
- thickening of the intestines that may be associated with abdominal pain, abdominal distension, or vomiting. In rare cases, EPS can be fatal
Children
If you are under 18 years of age, your doctor will carefully evaluate the benefit-risk of using this product.
Using PHYSIONEAL 40 and other medicines
- Tell your doctor if you are using or have recently used other medicines,
including those obtained without a prescription.
- If you use other medicines, your doctor may need to increase your dose since peritoneal dialysis treatment increases the elimination of certain medicines.
- Be careful if you use heart medications called cardiac glycosides (e.g., digoxin), you may:
- need potassium and calcium supplements
- develop cardiac arrhythmias.
- Your doctor will closely monitor you during treatment, especially your potassium levels.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. The use of Physioneal is not recommended during pregnancy or breastfeeding unless your doctor advises you to do so.
Driving and using machines
This treatment can cause weakness, blurred vision, or dizziness. Do not drive or use machines if you are affected.
3. How to use PHYSIONEAL 40
PHYSIONEAL 40 should be administered into your peritoneal cavity. This cavity is located in the
abdomen (belly) between the skin and the peritoneum. The peritoneum is the membrane that surrounds the internal organs, such as the intestines or liver.
Do not use intravenously.
Follow exactly the administration instructions of this medicine indicated by the medical team specialized in peritoneal dialysis. If in doubt, consult them again.
If the bag is damaged, it should be discarded.
Dose and frequency
Your doctor will indicate the appropriate glucose concentration and the number of bags you should use each day.
Use in children and adolescents
If you are under 18 years of age, your doctor will carefully evaluate the prescription of this
medicine.
If you stop treatment with PHYSIONEAL 40
Do not stop peritoneal dialysis without your doctor's consent. Stopping treatment can have harmful consequences for your life.
Method of administration
Before use,
- Warm the bag to 37 °C. Use the specially designed heating plate for this purpose. Never submerge the bag in water to heat it. Never use a microwave oven to heat the bag.
- You must use an aseptic technique during the entire administration of the solution, as you have been taught.
- Before performing an exchange, make sure to wash your hands and the area where you will perform the exchange.
- Before opening the overbag, check that it is the correct solution, the expiration date, and the quantity (volume). Lift the bag to check for leaks (excess liquid in the overbag). Do not use the bag if you find leaks.
- After removing the overbag, check for signs of leaks in the container by pressing firmly on the bag. Check that the breakable seal that separates the two chambers is not broken. If the seal is broken, discard the bag. Do not use the bag if you detect any leaks.
- Check that the solution is clear. Do not use the bag if the solution is cloudy or contains particles
- Before starting the exchange, check that all connections are secure.
- Mix the two chambers carefully by breaking the breakable seal between the two chambers. Wait until the upper chamber is completely empty into the lower chamber. Mix the solution carefully, pressing with both hands on the walls of the lower chamber.
- Consult your doctor if you have any questions or doubts about this product or its use.
- Use each bag only once. Discard any unused solution.
- The solution must be administered within 24 hours after mixing.
After use, check that the drainage fluid is not cloudy.
Compatibility with other medicines
Your doctor may prescribe other injectable medicines to be added directly to the PHYSIONEAL 40 bag. In this case, add the medicine through the medication addition site located in the small chamber, before breaking the breakable seal between the two chambers. Use the product immediately after adding the medicine. Consult your doctor if you are unsure.
If you use more PHYSIONEAL 40 bags in 24 hours than you should
If you are administered an excessive dose of PHYSIONEAL 40, you may experience:
- abdominal distension
- stomach heaviness and/or
- difficulty breathing.
Contact your doctor immediately. They will inform you of what to do.
If you have any further questions about the use of this product, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following, contact your doctor or peritoneal dialysis unit immediately:
- Hypertension (blood pressure above normal levels),
- inflammation of the ankles or legs, swollen eyes, difficulty breathing, or chest pain (hypervolemia),
- abdominal pain,
- chills (flu-like symptoms), fever.
- inflamed peritoneum (peritonitis)
These are serious side effects. You may need urgent medical attention.
If you experience any side effect, tell your doctor or peritoneal dialysis unit. This includes any possible side effect not listed in this leaflet.
Frequent (may affect up to 1 in 10 people):
- Changes in your blood parameters:
- increased calcium levels (hypercalcemia)
- decreased potassium levels (hypokalemia) that may cause muscle weakness, muscle contractions, or cardiac arrhythmias
- increased bicarbonate levels (alkalosis).
- weakness, fatigue
- fluid retention (edema)
- weight gain
Uncommon (may affect up to 1 in 100 people):
- decreased fluid removal during dialysis.
- fainting, dizziness, or headache.
- cloudy solution extracted from the peritoneum, stomach pain.
- peritoneal hemorrhage, pus, swelling, or pain around the catheter exit site, and catheter blockage.
- nausea, loss of appetite, indigestion, flatulence (gas), thirst, and dry mouth.
- abdominal distension or inflammation, shoulder pain, abdominal cavity hernia (bulge in the groin).
- changes in your blood parameters:
- lactic acidosis
- increased carbon dioxide levels
- increased sugar levels (hyperglycemia)
- increased white blood cell count (eosinophilia)
- difficulty sleeping
- low blood pressure (hypotension)
- cough
- muscle or bone pain
- facial or throat inflammation
- skin rash
Other side effects related to the peritoneal dialysis procedure:
- infection around the catheter exit site, catheter blockage
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of PHYSIONEAL 40
- Keep this medicine out of the sight and reach of children.
- Store in the original packaging.
- Do not store at a temperature below 4 °C.
- Do not use this medicine after the expiration date stated on the outer packaging label and on the bag after the abbreviation EXP and the symbol.
Discard PHYSIONEAL 40 as indicated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
This leaflet does not contain all the information about this medicine. If you have any doubts or are unsure about something, ask your doctor.
Composition of PHYSIONEAL 40
The mixed peritoneal dialysis solution contains the following active ingredients:
1.36% | 2.27% | 3.86% | |
Glucose monohydrate (g/l) | 15.0 | 25.0 | 42.5 |
equivalent to anhydrous glucose (g/l) | 13.6 | 22.7 | 38.6 |
Sodium chloride (g/l) | 5.38 | ||
Calcium chloride dihydrate (g/l) | 0.184 | ||
Magnesium chloride hexahydrate (g/l) | 0.051 | ||
Sodium bicarbonate (g/l) | 2.10 | ||
Sodium L-lactate solution (g/l) | 1.68 |
The other components are: water for injectable preparations and carbon dioxide.
The composition in mmol/l of the mixed solution is:
1.36% | 2.27% | 3.86% | |
Anhydrous glucose (mmol/l) | 75.5 | 126 | 214 |
Sodium (mmol/l) | 132 | ||
Calcium (mmol/l) | 1.25 | ||
Magnesium (mmol/l) | 0.25 | ||
Chloride (mmol/l) | 95 | ||
Bicarbonate (mmol/l) | 25 | ||
Lactate (mmol/l) | 15 |
Appearance of the Product and Package Contents
PHYSIONEAL 40 is a colorless, transparent, and sterile solution for peritoneal dialysis.
- PHYSIONEAL 40 is packaged in a double-chamber PVC bag. The two chambers are separated by a permanent seal. You should only administer PHYSIONEAL 40 when the solutions from the two chambers are fully mixed.
- Each bag is wrapped in an overbag and supplied in a cardboard box.
Volume | Number of units per box | Product presentation | Types of connectors |
1.51 | 5 / 6 | Single bag (DPA) | luer |
1.51 | 5 / 6 | Double bag (DPCA) | luer |
2.01 | 4 / 5 | Single bag (DPA) | luer |
2.01 | 4 / 5 | Double bag (DPCA) | luer |
2.51 | 4 / 5 | Single bag (DPA) | luer |
2.51 | 4 / 5 | Double bag (DPCA) | luer |
Only some package sizes may be marketed.
Marketing Authorization Holder
Vantive Health, S.L.
Polígono Industrial Sector 14
C/Pouet de Camilo 2
46394 Ribarroja del Turia (Valencia) Spain
Manufacturer
Vantive Manufacturing Limited
Moneen Road
Castlebar
County Mayo – Ireland
or
Bieffe Medital SpA
Via Nuova Provinciale
23034 Grosotto
Italy
Date of the last revision of this leaflet: November 2018
Detailed and updated information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Vantive and Physioneal are registered trademarks of Vantive Health Inc. or its affiliates
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PHYSIONEAL 40 3.86% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS 38.6 mg/mlDosage form: PERITONEAL DIALYSIS, 1000 mlActive substance: Hypertonic solutionsManufacturer: Fresenius Medical Care Deutschland GmbhPrescription requiredDosage form: PERITONEAL DIALYSIS, 1000 mlActive substance: Hypertonic solutionsManufacturer: Fresenius Medical Care Deutschland GmbhPrescription requiredDosage form: PERITONEAL DIALYSIS, 4.25 %Active substance: Hypertonic solutionsManufacturer: Fresenius Medical Care Deutschland GmbhPrescription required
Online doctors for PHYSIONEAL 40 3.86% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS 38.6 mg/ml
Discuss questions about PHYSIONEAL 40 3.86% GLUCOSE SOLUTION FOR PERITONEAL DIALYSIS 38.6 mg/ml, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















