
PEGASYS 135 micrograms, pre-filled syringe injectable solution

How to use PEGASYS 135 micrograms, pre-filled syringe injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet:information for the user
Pegasys 90micrograms solution for injection in pre-filled syringe
Pegasys 135micrograms solution for injection in pre-filled syringe
Pegasys 180micrograms solution for injection in pre-filled syringe
peginterferon alfa-2a
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Pegasys and what is it used for
- What you need to know before you use Pegasys
- How to use Pegasys
- Possible side effects
- Storage of Pegasys
- Contents of the pack and other information
1. What is Pegasys and what is it used for
Pegasys contains the active substance peginterferon alfa-2a, which is a long-acting interferon. Interferon is a protein that modifies the immune system's response to help fight infections and serious diseases and to prevent the growth of cancer cells.
Pegasys is used to treat polycythaemia vera and essential thrombocythaemia in adults.
Polycythaemia vera and essential thrombocythaemia are rare types of cancer in which the bone marrow produces too many red blood cells, white blood cells, and platelets (cells that help the blood to clot).
Polycythaemia vera and essential thrombocythaemia:Pegasys is used alone.
Pegasys is also used to treat chronic hepatitis B or C in adults. It is also used to treat chronic hepatitis B in children and adolescents from 3 years of age and chronic hepatitis C in children and adolescents from 5 years of age who have not been treated before. Both hepatitis B and C are viral infections that affect the liver.
Chronic hepatitis B:Pegasys is usually used alone.
Chronic hepatitis C:Pegasys is used in combination with other medicines for the treatment of chronic hepatitis C (CHC).
You should also read the package leaflet of the medicines used in combination with Pegasys.
2. What you need to know before you use Pegasys
Do not use Pegasys
- if you are allergic to peginterferon alfa-2a, to any interferon, or to any of the other ingredients of this medicine (listed in section 6).
- if you have had a heart attack or have been hospitalized for severe chest pain in the last 6 months.
- if you have or have had an autoimmune disease (such as rheumatoid arthritis, psoriasis, or inflammatory bowel disease).
- if you have an uncontrolled thyroid disease.
- if you have advanced liver disease and your liver is not working properly (e.g., your skin has turned yellow).
- if the patient is under 3 years of age.
- if the patient is a child who has had severe psychiatric disorders such as severe depression or suicidal thoughts.
- if you are infected with hepatitis C virus and human immunodeficiency virus, and your liver is not working properly (e.g., your skin has turned yellow).
- if you are receiving treatment with telbivudine, a medicine for hepatitis B virus infection (see “Do not use Pegasys”).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Pegasys
- if you have had a severe mental or nervous disorder.
- if you have had depression or symptoms related to depression (e.g., feeling sad, discouraged, etc.).
- if you are an adult with a history of substance abuse (e.g., alcohol or drugs).
- if you have psoriasis, it may worsen during treatment with Pegasys.
- if you have other liver problems, apart from hepatitis B or C.
- if you have diabetes or high blood pressure, your doctor may ask for an eye examination.
- if you have been told you have Vogt-Koyanagi-Harada syndrome.
- if you have an uncontrolled thyroid disease.
- if you have had anaemia.
- if you have had an organ transplant (liver or kidney) or are planning to have one in the near future.
- if you are co-infected with human immunodeficiency virus (HIV) and are being treated with anti-HIV medicines.
- if you have been taken off previous treatment for hepatitis C due to anaemia or low blood count.
Once you have started treatment with Pegasys, talk to your doctor, nurse, or pharmacist:
- if you develop symptoms related to depression (e.g., feeling sad, discouraged, etc.) (see section 4).
- if you notice any changes in your vision.
- if you have symptoms associated with a cold or other respiratory infection (such as cough, fever, or difficulty breathing).
- if you think you are getting an infection (such as pneumonia) since when you are being treated with Pegasys, you may have a temporarily increased risk of getting infections.
- if you have any signs of bleeding or unusual bruising, consult your doctor immediately.
- if you have signs of a severe allergic reaction (such as difficulty breathing, wheezing, or hives) while you are being treated with this medicine, seek medical help immediately.
- if you have symptoms of Vogt-Koyanagi-Harada syndrome; a combination of symptoms such as stiff neck, headache, loss of skin or hair colour, eye disorders (such as blurred vision), and/or hearing disorders (such as ringing in the ears).
During treatment, your doctor will take blood samples from you regularly to check for changes in your white blood cells, red blood cells, platelets, liver function, glucose (blood sugar levels), or changes in other laboratory values.
Disorders of the teeth and gums that can lead to tooth loss have been reported in patients treated with Pegasys in combination with ribavirin. Additionally, dry mouth could have harmful effects on teeth and mouth mucosa in long-term treatments with Pegasys in combination with ribavirin. You should brush your teeth properly twice a day and have regular dental check-ups. Some patients may also suffer from vomiting. If you have this reaction, you should rinse your mouth properly afterwards.
Children and adolescents
Indication for polycythaemia vera and essential thrombocythaemia:
Pegasys must not be given to children and adolescents because there is no information available on the use of Pegasys in this age group for these indications.
Indication for chronic hepatitis B and C:
Pegasys is restricted to children and adolescents with chronic hepatitis C from 5 years of age or children and adolescents with chronic hepatitis B from 3 years of age. Pegasys must not be given to children under 3 years of age because it contains benzyl alcohol and may cause toxic and allergic reactions in these children.
- If your child has or has had a psychiatric disorder, talk to your doctor, who will monitor your child for signs and symptoms of depression (see section 4).
- While your child is receiving Pegasys, they may have slower growth and development (see section 4).
Other medicines and Pegasys
Do not use Pegasys if you are taking telbivudine (see “Do not use Pegasys”) because the combination of these medicines increases the risk of developing peripheral neuropathy (numbness, tingling, and/or burning sensation in the arms and/or legs). Therefore, the combination of Pegasys with telbivudine is contraindicated. Tell your doctor or pharmacist if you are receiving treatment with telbivudine.
Tell your doctor if you are taking any medicine for asthma as it may be necessary to change the dose of your asthma medication.
Patients who are also infected with HIV: Tell your doctor if you are taking anti-HIV treatment. Lactic acidosis and worsening of liver function are side effects associated with highly active antiretroviral therapy (HAART), an HIV treatment. If you are receiving HAART, the addition of Pegasys + ribavirin may increase the risk of lactic acidosis or liver failure. Your doctor will monitor you for signs and symptoms of these conditions. Patients who have received zidovudine in combination with ribavirin and interferon alfa may have an increased risk of developing anaemia. Patients who are receiving azathioprine in combination with ribavirin and peginterferon have a higher risk of developing severe blood disorders. Please also read the package leaflet of ribavirin.
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
When Pegasys is used in combination with ribavirin, both male and female patients must take special precautions in their sexual activities if there is a risk of pregnancy because ribavirin can be very harmful to the unborn baby.
- If you are a womanof childbearing potential and are taking Pegasys in combination with ribavirin, you must have a pregnancy test and this must be negative before you start treatment, every month during treatment, and for 4 months after treatment has finished. You must use effective contraception during treatment and for 4 months after finishing treatment. You can discuss this with your doctor.
- If you are a manand are taking Pegasys in combination with ribavirin, you must not have sex with a pregnant woman unless you use a condom. This will reduce the chance of ribavirin being released into the woman's body. If your partner is not pregnant but is of childbearing potential, she must have a pregnancy test every month during treatment and for 7 months after treatment has finished. You or your partner must use effective contraception during the time you are using treatment and for 7 months after treatment has finished. You can discuss this with your doctor.
Ask your doctor or pharmacist for advice before taking any medicine. It is not known if this product is present in human milk. Therefore, you must not breast-feed if you are being treated with Pegasys. In combination therapy with ribavirin, pay attention to the corresponding information for medicines containing ribavirin.
You should also read the package leaflet of the medicines used in combination with Pegasys.
Driving and using machines
Do not drive or use machines if you feel drowsy, tired, or confused while you are being treated with Pegasys.
Pegasys containsbenzyl alcohol,polysorbate80, and sodium
This medicine contains 5 mg of benzyl alcohol in each pre-filled syringe, which is equivalent to 10 mg/ml.
Benzyl alcohol may cause allergic reactions.
Benzyl alcohol has been linked to the risk of serious side effects, including respiratory problems (called “gasping syndrome”) in small children. Pegasys must not be given to premature newborns, newborns at term, or children up to 3 years of age.
Ask your doctor or pharmacist if you are pregnant or breast-feeding, or if you have any liver or kidney disease. This is because large amounts of benzyl alcohol may build up in your body and cause side effects (called “metabolic acidosis”).
This medicine contains 0.025 mg of polysorbate 80 in each pre-filled syringe, which is equivalent to 0.05 mg/ml. Polysorbates may cause allergic reactions. Tell your doctor if you or your child have any known allergies.
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially “sodium-free”.
3. How to use Pegasys
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, consult your doctor or pharmacist again.
Dosage of Pegasys
Your doctor has decided on the exact dose of Pegasys, and will tell you how often you should use it. If necessary, they will change the dose during treatment. Do not exceed the recommended dose.
Polycythaemia vera and essential thrombocythaemia in adults
Pegasys for treating polycythaemia vera and essential thrombocythaemia is given alone with the recommended initial dose of 45 micrograms once a week by subcutaneous injection and is usually adjusted in increments of 45 micrograms once a month up to a maximum of 180 micrograms once a week by subcutaneous injection.
Your doctor may adjust the dose and/or prolong the administration interval.
Hepatitis B and C in adults
Pegasys is used alone (as the only treatment), only if for some reason you cannot take ribavirin.
Pegasys, alone or in combination with ribavirin, is usually given in doses of 180 micrograms once a week. See also the sections below for combination treatments.
The duration of combination treatment varies from 4 to 18 months depending on the type of virus you are infected with, your response during treatment, and whether you have been treated before.
Please consult your doctor and follow the recommended treatment duration.
The injection of Pegasys is usually given at bedtime.
Use in children and adolescents
Hepatitis B (from 3 years of age) and hepatitis C (from 5 years of age)
Your doctor has determined the exact dose of Pegasys for your child and will inform you how often they should use it. The usual dose of Pegasys is based on your child's height and weight. If necessary, the dose may be changed during treatment. For children and adolescents, it is recommended to use Pegasys pre-filled syringes, as they allow for dose adjustment. Do not exceed the recommended dose.
The duration of combination treatment in children with chronic hepatitis C varies from 6 to 12 months, depending on the type of virus your child is infected with and their response to treatment. The duration of treatment with Pegasys in chronic hepatitis B is 48 weeks. Consult your doctor and follow the recommended treatment duration. The injection of Pegasys is usually given at bedtime.
Pegasys is given by subcutaneous injection (under the skin). This means that Pegasys is injected with a short needle into the fatty tissue under the skin of the abdomen or thigh. If you are going to inject this medicine yourself, you must be instructed on how to do it. Detailed instructions are attached at the end of the package leaflet (see “How to inject Pegasys”).
Use Pegasys exactly as your doctor has told you and for as long as they have told you. If you think the effect of Pegasys is too strong or too weak, tell your doctor or pharmacist.
Combination treatment with ribavirin in chronic hepatitis C(all patients from 5 years of age)
In the case of combination treatment of Pegasys and ribavirin, please follow the recommended dosing regimen as told by your doctor.
Combination treatment with other medicines in chronic hepatitis C(all patients from 5 years of age)
In the case of combination treatment with Pegasys, please follow the recommended dosing regimen as told by your doctor and you should also read the package leaflet of the medicines used in combination with Pegasys.
If you use more Pegasys than you should
Contact your doctor or pharmacist as soon as possible.
If you forget to use Pegasys
If you realize you have forgotten the injection 1 or 2 days after it was due,inject the recommended dose as soon as possible. Continue with the next injection following the planned schedule.
If you realize you have forgotten the injection 3 to 5 days after it was due, inject the recommended dose as soon as possible and then inject the next doses at 5-day intervals until you recover the weekly schedule.
Example: you inject Pegasys every Monday. However, on Friday, you realize you forgot the injection due on the previous Monday (delay of 4 days). You should inject the regular dose immediately on Friday and the next one on the following Wednesday (5 days after the Friday dose). The next injection will be on Monday, 5 days after Wednesday. You have now recovered your old weekly schedule and can continue injecting every Monday.
If you realize you have forgotten the injection 6 days after it was due,you should wait and inject the dose the next day to maintain your usual schedule.
Consult your doctor or pharmacist if you need more detailed information in case you forget a dose of Pegasys.
Do not take a double dose to make up for forgotten doses.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Some people may suffer from depression when treated with Pegasys alone or in combination with ribavirin, and in some cases have had suicidal thoughts or aggressive behavior (sometimes directed towards others, such as the idea of attacking the life of others). In fact, some patients have committed suicide. Make sure to seek emergency care if you feel that you are becoming depressed or have suicidal thoughts or changes in your behavior. You may want to consider asking a close family member or friend to help you stay alert to signs of depression or changes in your behavior. |
Growth and Development (Children and Adolescents) Some children and adolescents treated with Pegasys for chronic hepatitis B for 48 weeks did not grow or gain weight as expected for their age. It is still unknown whether they will return to their expected height and weight after completing treatment. During the year of treatment with Pegasys in combination with ribavirin, some children and adolescents with chronic hepatitis C did not grow or gain as much weight as expected. Most children reached the expected height during the two years after finishing treatment, and the rest of the children within six years after completing treatment; it is possible that Pegasys may affect their final adult height. |
Talk to your doctor immediately if you have any of these adverse reactions: severe chest pain, persistent cough, irregular heartbeats, breathing problems, confusion, depression, severe stomach pain, blood in stools (or black tarry stools), severe nosebleeds, fever or chills, vision problems. These adverse effects can be serious and you may need urgent medical attention.
The very common adverse effects of Pegasys in combination with ribavirin (may affect more than 1 in 10 people) are:
Metabolic disorders: loss of appetite
Psychiatric and nervous system disorders: depressive feelings (feeling sad, low mood, pessimism), anxiety, insomnia, headache, difficulty concentrating, and dizziness
Respiratory disorders: cough, difficulty breathing
Digestive disorders: diarrhea, nausea, abdominal pain
Skin disorders: hair loss and skin reactions (including itching, dermatitis, and dry skin)
Musculoskeletal and bone disorders: joint pain and muscle pain
General disorders: fever, weakness, fatigue, tremors, chills, pain, irritation at the injection site, and irritability (easily irritated)
The common adverse effects of Pegasys in combination with ribavirin (may affect up to 1 in 10 people) are:
Infections: fungal, viral, and bacterial infections. Upper respiratory tract infections, bronchitis, fungal infections in the mouth, and herpes (a common and recurrent viral infection that affects the lips and mouth)
Blood disorders: low platelet count (affects blood clotting), anemia (low red blood cell count), and lymph node inflammation
Hormonal system disorders: high and low thyroid activity
Psychiatric and nervous system disorders: mood changes/emotional changes, aggression, nervousness, decreased sexual desire, memory loss, fainting, decreased muscle tone, migraine, numbness, tingling, feeling of heat, tremor, alteration of taste, nightmares, somnolence
Eye disorders: blurred vision, eye pain, eye inflammation, and dry eyes
Ear disorder: ear pain
Heart and blood vessel disorders: rapid heartbeat, palpitations, inflammation of the limbs, flushing
Respiratory disorders: difficulty breathing when exercising, nosebleeds, nasal and throat inflammation, nasal and sinus infections, nasal discharge, sore throat
Digestive disorders: vomiting, indigestion, difficulty swallowing, mouth ulcers, gum bleeding, tongue and mouth inflammation, flatulence (excess air or gas), dry mouth, and weight loss
Skin disorders: rash, increased sweating, psoriasis, skin redness and itching (urticaria), eczema, sensitivity to light, night sweats
Musculoskeletal and bone disorders: back pain, joint inflammation, muscle weakness, bone pain, neck pain, muscle pain, muscle cramps
Reproductive system disorders: impotence (inability to maintain an erection)
General disorders: chest pain, flu-like symptoms, discomfort, lethargy, hot flashes, thirst
The uncommon adverse effects of Pegasys in combination with ribavirin (may affect up to 1 in 100 people) are:
Infections: pneumonia, skin infections
Benign and malignant neoplasms: liver tumor
Immune system disorders: sarcoidosis (inflamed tissue areas throughout the body), thyroid inflammation
Hormonal system disorders: diabetes (high blood sugar levels)
Metabolic disorders: dehydration
Psychiatric and nervous system disorders: suicidal thoughts, hallucinations, peripheral neuropathy (nerve disorders affecting the limbs)
Eye disorders: retinal hemorrhage (back part of the eye)
Ear disorder: hearing loss
Heart and blood vessel disorders: high blood pressure
Respiratory disorders: wheezing (whistling sounds when breathing)
Digestive disorders: gastrointestinal bleeding
Liver disorders: liver dysfunction
The rare adverse effects of Pegasys in combination with ribavirin (may affect up to 1 in 1,000 people) are:
Infections: heart infection, external ear infection
Blood disorders: severe decrease in red blood cells, white blood cells, and platelets
Immune system disorders: severe allergic reactions, systemic lupus erythematosus (the body attacks its own cells), rheumatoid arthritis (an autoimmune disease)
Hormonal system disorders: diabetic ketoacidosis, a complication of uncontrolled diabetes
Psychiatric and nervous system disorders: suicide, psychotic disorders (serious personality problems and deterioration in normal social functioning), coma (deep and prolonged unconsciousness), seizures, facial paralysis (weakness of facial muscles)
Eye disorders: optic nerve inflammation, retinal inflammation, corneal ulcer
Heart and blood vessel disorders: heart attack, heart failure, chest pain, rapid heartbeat, cardiac rhythm disorder, or inflammation of the heart lining and heart muscle, cerebral hemorrhage, and inflammation in the vessels
Respiratory disorders: interstitial pneumonia (inflammation of the lungs, including fatal outcome), blood clots in the lungs
Digestive disorders: gastric ulcer, pancreas inflammation
Liver disorders: liver failure, bile duct inflammation, fatty liver
Musculoskeletal and bone disorders: muscle inflammation
Kidney disorders: kidney failure
Trauma and poisoning: overdose
The very rare adverse effects of Pegasys in combination with ribavirin (may affect up to 1 in 10,000 people) are:
Blood disorders: aplastic anemia (bone marrow failure to produce red blood cells, white blood cells, and platelets)
Immune system disorders: idiopathic or thrombocytopenic purpura (increase in bruising, bleeding, decrease in platelets, anemia, and extreme weakness)
Eye disorders: vision loss
Skin disorders: toxic epidermal necrolysis/Stevens-Johnson syndrome/erythema multiforme (a range of rashes with various degrees of severity that can be life-threatening and may be associated with blisters in the mouth, nose, eyes, and other mucous membranes and shedding of the affected skin area), angioedema (inflammation of the skin and mucous membranes)
Adverse effects of unknown frequency:
Blood disorders: pure red cell aplasia (a severe type of anemia in which red blood cell production is decreased or stopped), which can lead to symptoms such as feeling very tired and lacking energy
Immune system disorders: Vogt-Koyanagi-Harada disease, a rare disease characterized by vision loss, hearing loss, and skin pigmentation; liver and kidney transplant rejection
Psychiatric and nervous system disorders: mania (episodes of exaggerated mood elevation) and bipolar disorders (episodes of exaggerated mood elevation alternating with sadness and despair); thoughts of threatening the life of others, stroke (lack of blood flow to the brain)
Eye disorders: a rare form of retinal detachment with fluid in the retina
Heart and blood vessel disorders: peripheral ischemia (insufficient blood supply to the limbs)
Digestive disorders: ischemic colitis (insufficient blood supply to the intestine), changes in tongue color
Musculoskeletal and bone disorders: severe muscle damage and pain
Pulmonary arterial hypertension is a disease in which there is a significant narrowing of the blood vessels in the lungs, causing an increase in pressure in the blood vessels that carry blood from the heart to the lungs. This can occur especially in patients with risk factors such as HIV infection or severe liver problems (cirrhosis). Episodes were reported at different times, even several months after starting treatment with Pegasys.
If Pegasys is administered alone in patients with hepatitis B or C, the likelihood of these effects is reduced.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Pegasys
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the pre-filled syringe in the original packaging to protect it from light.
Do not use this medicine if you notice that the syringe or needle container is damaged, if the solution is cloudy, has particles floating, or has a color other than colorless to light yellow.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Pegasys Composition
- The active ingredient is peginterferon alfa-2a. Each 0.5 ml pre-filled syringe contains 90, 135, or 180 micrograms of peginterferon alfa-2a.
- The other ingredients are sodium chloride, polysorbate 80, benzyl alcohol, sodium acetate, acetic acid, and water for injectable preparations.
Product Appearance and Package Contents
Pegasys is presented as an injectable solution contained in a pre-filled syringe (0.5 ml) with a separate injection needle.
Pegasys 90 micrograms injectable solution in pre-filled syringe
The syringe has graduation marks corresponding to 90 micrograms (µg), 65 µg, 45 µg, 30 µg, 20 µg, and 10 µg. It is available in packs of 1 pre-filled syringe.
Pegasys 135 micrograms injectable solution in pre-filled syringe
The syringe has graduation marks corresponding to 135 micrograms (µg), 90 µg, and 45 µg. It is available in packs of 1, 4, or a multiple pack containing 12 (2 packs of 6) pre-filled syringes. Not all pack sizes may be marketed.
Pegasys 180 micrograms injectable solution in pre-filled syringe
The syringe has graduation marks corresponding to 180 micrograms (µg), 135 µg, and 90 µg. It is available in packs of 1, 4, or a multiple pack containing 12 (2 packs of 6) pre-filled syringes. Not all pack sizes may be marketed.
Marketing Authorization Holder
pharmaand GmbH
Taborstrasse 1
1020 Wien
Austria
Manufacturer
Loba biotech GmbH
Fehrgasse 7
2401 Fischamend
Austria
Date of Last Revision of this Prospectus:
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
How to Inject Pegasys
The following instructions explain how to use the Pegasys pre-filled syringes to inject yourself or your child. Please read the instructions carefully and follow them step by step. Your doctor or other healthcare professional will give you instructions on how to administer the injections.
Initial Preparation
Wash your hands carefully before handling any of the materials.
Have everything you need ready before you start:
Included in the Package:
- a Pegasys pre-filled syringe
- an injection needle
Not Included in the Package:
- a cotton ball for cleaning
- a small bandage or sterile gauze
- a sticky plaster
- a container for disposing of waste
Preparing the Syringe and Needle for Injection
- Remove the protective cap that covers the end of the needle (1-2).
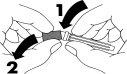
- Remove the rubber cap from the syringe (3). Do not touch the tip of the syringe.
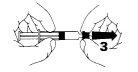
- Firmly attach the needle to the tip of the syringe (4).
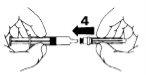
- Remove the needle protector (5).
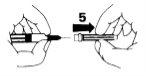
- To eliminate air bubbles from the syringe, hold it in a vertical position with the needle facing up. Tap the syringe several times with your finger to make the bubbles rise to the top of the liquid. Slowly push the plunger until you reach the correct dose, where the edge of the plunger comes into contact with the syringe. Put the needle protector back on and place the syringe in a horizontal position until you are ready to use it.
- Allow the solution to reach room temperature before injection or warm the syringe by holding it between your hands.
- Visually inspect the solution before administration: do not use it if it has changed color or if you notice that it has particles.
Now you are ready to inject the dose.
Injecting the Solution
- Choose the injection site on the abdomen or thigh (except the navel or waist area). Change the injection site each time you administer it.
- Clean and disinfect the skin in the area where you will inject yourself with a cotton ball.
- Wait for the area to dry.
- Remove the needle protector.
- With one hand, pinch the skin, and with the other hand, hold the syringe like a pen.
- Insert the needle completely at an angle of 45° to 90° into the skin that you have pinched with the other hand (6).
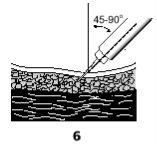
- Inject the solution by pushing the plunger down from the appropriate graduation.
- Remove the needle from the skin.
- If necessary, press the injection site with a small bandage or sterile gauze for a few seconds.
Do not massage the injection site. If it bleeds, cover it with a sticky plaster.
Disposal of Injection Materials
The syringe, needle, and all injection materials are for single use and must be disposed of after injection. Dispose of the syringe and needle safely in a closed container. Ask your doctor, hospital, or pharmacist for a suitable container.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PEGASYS 135 micrograms, pre-filled syringe injectable solutionDosage form: INJECTABLE, 90 mcg / 0.5 mlActive substance: peginterferon alfa-2aManufacturer: Pharmaand GmbhPrescription requiredDosage form: INJECTABLE, UnknownActive substance: peginterferon alfa-2aManufacturer: Pharmaand GmbhPrescription requiredDosage form: INJECTABLE, UnknownActive substance: peginterferon alfa-2aManufacturer: Pharmaand GmbhPrescription required
Online doctors for PEGASYS 135 micrograms, pre-filled syringe injectable solution
Discuss questions about PEGASYS 135 micrograms, pre-filled syringe injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















