OSVYRTI 60 mg Injectable Solution in Pre-filled Syringe

How to use OSVYRTI 60 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Osvyrti 60 mg solution for injection in pre-filled syringe
denosumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- Your doctor will provide you with a patient reminder card, which contains important safety information that you should know before and during treatment with Osvyrti.
Contents of the pack
- What Osvyrti is and what it is used for
- What you need to know before you use Osvyrti
- How to use Osvyrti
- Possible side effects
- Storage of Osvyrti
- Contents of the pack and other information
1. What Osvyrti is and what it is used for
What Osvyrti is and how it works
OSVYRTI contains denosumab, a protein (monoclonal antibody) that interferes with the action of another protein in order to treat bone loss and osteoporosis. Treatment with Osvyrti strengthens bones and reduces the risk of fractures.
Bone is a living tissue that is constantly renewed. Estrogens contribute to the preservation of bone health. After menopause, estrogen levels decrease, which can cause bones to become thinner and more fragile. In the long run, this can lead to a disease called osteoporosis. Osteoporosis can also occur in men due to various causes, including age and/or low levels of the male hormone, testosterone. It can also occur in patients undergoing treatment with glucocorticoids. Many patients with osteoporosis do not have symptoms, although they still have a risk of fracturing bones, especially in the spine, hip, and wrists.
Surgical interventions or medications that stop the production of estrogen or testosterone, used to treat patients with prostate or breast cancer, can also cause bone loss. This makes bones weaker and more prone to breaking.
What Osvyrti is used for
Osvyrti is used to treat:
- postmenopausal osteoporosis in women and in men who have an increased risk of fracture (bone breakage), reducing the risk of fractures of the hip, spine, and non-spinal locations.
- bone loss caused by reduced hormonal levels (testosterone) as a result of surgical intervention or treatment with medications in patients with prostate cancer.
- bone loss resulting from long-term treatment with glucocorticoids in patients who have a high risk of fracture.
2. What you need to know before you use Osvyrti
Do not use Osvyrti:
- if you have low levels of calcium in the blood (hypocalcemia).
- if you are allergic to denosumab or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Osvyrti.
During treatment with Osvyrti, you may develop a skin infection with symptoms such as an inflamed and reddened area on the skin, more frequently on the lower leg, which feels hot and sensitive to the touch (cellulitis), and may be accompanied by fever. Inform your doctor immediately if you experience any of these symptoms.
In addition, you should take calcium and vitamin D supplements during treatment with Osvyrti. Your doctor will discuss this with you.
While receiving Osvyrti, you may experience low levels of calcium in the blood. Inform your doctor immediately if you notice any of the following symptoms: muscle spasms, contractions, or cramps, and/or numbness or tingling in the fingers of the hands, feet, or around the mouth, and/or convulsions, confusion, or loss of consciousness.
In rare cases, very low levels of calcium in the blood have been reported, which have required hospitalization and, in some cases, potentially life-threatening reactions. Therefore, before each dose is administered and, in patients with a predisposition to hypocalcemia, within two weeks after the initial dose, your blood calcium levels will be checked (through a blood test).
Inform your doctor if you have or have had severe kidney problems, kidney failure, if you have needed to undergo dialysis, or if you are taking medications called glucocorticoids (such as prednisolone or dexamethasone), as they may increase the risk of having low levels of calcium in the blood if you do not take calcium supplements.
Problems in the mouth, teeth, or jaw
In patients receiving Osvyrti for osteoporosis, a rare side effect called osteonecrosis of the jaw (ONJ) (damage to the jawbone) has been reported (may affect up to 1 in 1,000 people). The risk of ONJ increases in patients treated for a long time (may affect up to 1 in 200 people if treated for 10 years). ONJ can also occur after treatment is stopped. It is essential to try to prevent the development of ONJ, as it can be a painful condition that can be difficult to treat. To reduce the risk of developing ONJ, follow these precautions:
Before receiving treatment, inform your doctor or nurse (healthcare professional) if:
- you have any problems in your mouth or teeth, such as poor dental health, gum disease, or a planned tooth extraction.
- you do not receive regular dental check-ups or have not had a dental check-up for a long time.
- you are a smoker (as this may increase the risk of dental problems).
- you have been previously treated with a bisphosphonate (used to prevent or treat bone disorders).
- you are taking medications called corticosteroids (such as prednisolone or dexamethasone).
- you have cancer.
Your doctor may ask you to undergo a dental check-up before starting treatment with Osvyrti.
During treatment with Osvyrti, you should maintain good oral hygiene and undergo routine dental check-ups. If you use dental prosthetics, you should ensure that they fit properly. If you are undergoing dental treatment or are going to undergo dental surgery (e.g., tooth extractions), inform your doctor about your dental treatment and inform your dentist that you are being treated with Osvyrti.
Contact your doctor and dentist immediately if you experience any problems in your mouth or teeth, such as loose teeth, pain, or inflammation, or ulcers that do not heal or are suppurating, as these could be symptoms of ONJ.
Unusual fractures of the thigh
Some people have developed unusual fractures in the thigh while being treated with Osvyrti. Consult your doctor if you experience new or unusual pain in the hip, groin, or thigh.
Children and adolescents
Osvyrti should not be used in children and adolescents under 18 years of age.
Using Osvyrti with other medicines
Inform your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. It is especially important that you inform your doctor if you are being treated with another medicine that contains denosumab.
Do not use Osvyrti with another medicine that contains denosumab.
Pregnancy and breastfeeding
Osvyrti has not been tested in pregnant women. It is essential that you inform your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant. Osvyrti is not recommended during pregnancy. Women of childbearing age should use effective contraceptive methods during treatment with Osvyrti and for at least 5 months after stopping treatment with Osvyrti.
If you become pregnant during treatment with Osvyrti or less than 5 months after stopping treatment with Osvyrti, inform your doctor.
It is not known whether Osvyrti is excreted in breast milk. It is essential that you inform your doctor if you are breastfeeding or plan to breastfeed. Your doctor will help you decide whether to stop breastfeeding or stop using Osvyrti, considering the benefit of breastfeeding for the child and the benefit of Osvyrti for the mother.
If you are breastfeeding during treatment with Osvyrti, please inform your doctor.
Consult your doctor or pharmacist before using any medicine.
Driving and using machines
Osvyrti has no or negligible influence on the ability to drive and use machines.
Osvyrti contains sorbitol
This medicine contains 46 mg of sorbitol per ml of solution.
Osvyrti contains polysorbate 20
This medicine contains 0.1 mg of polysorbate 20 in each 1 ml pre-filled syringe. Polysorbates may cause allergic reactions. Inform your doctor if you have known allergies.
Osvyrti contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 60 mg; this is essentially "sodium-free".
3. How to use Osvyrti
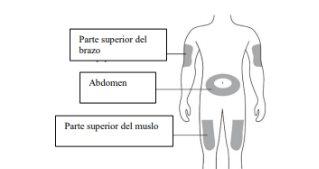
The recommended dose is a 60 mg pre-filled syringe administered under the skin (subcutaneously) in a single injection once every 6 months. The best places for injection are the top of the thighs and the abdomen. If the injection is given by a caregiver (the person who takes care of you), the injection can also be given in the outer aspect of the upper arm. Consult your doctor for the date of the next possible injection. Each pack of Osvyrticontains a removable label that can be detached from the blister pack and used to keep a record of the date of the next injection.
In addition, you should take calcium and vitamin D supplements during treatment with Osvyrti. Your doctor will discuss this with you.
Your doctor may decide whether it is best for you or a caregiver to administer the Osvyrti injection. Your doctor or healthcare professional will show you or your caregiver how to use Osvyrti. If you want to obtain instructions on how to inject Osvyrti, read the last section of this leaflet.
Do not shake.
If you miss a dose of Osvyrti
If you miss a dose of Osvyrti, the injection should be given as soon as possible. Subsequent injections should be scheduled every 6 months from the date of the last injection.
If you stop treatment with Osvyrti
To get the most benefit from your treatment and reduce the risk of fractures, it is essential that you use Osvyrti for the entire period prescribed by your doctor. Do not stop treatment without talking to your doctor first.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Patients treated with Osvyrti may develop skin infections (mainly cellulitis) infrequently. Inform your doctor immediatelyif you experience any of these symptoms during treatment with Osvyrti: an inflamed and reddened area on the skin, usually on the lower leg, which feels hot and sensitive to the touch and may be accompanied by fever.
Rarely, patients receiving Osvyrti may develop pain in the mouth and/or jaw, inflammation, or ulcers that do not heal in the mouth or jaw, suppurating, numbness, or a feeling of heaviness in the jaw, or tooth mobility. These could be symptoms of bone damage in the jaw (osteonecrosis). Inform your doctor and dentist immediatelyif you experience such symptoms while being treated with Osvyrti or after stopping treatment.
Rarely, patients receiving Osvyrti may experience low levels of calcium in the blood (hypocalcemia). Symptoms include muscle spasms, contractions, or cramps, and/or numbness or tingling in the fingers of the hands, feet, or around the mouth, and/or convulsions, confusion, or loss of consciousness. If you experience any of these, inform your doctor immediately. Low levels of calcium in the blood can also cause a change in heart rhythm called QT prolongation, which can be observed by performing an electrocardiogram (ECG).
Rarely, unusual fractures of the thigh may occur in patients receiving Osvyrti. Consult your doctorif you experience new or unusual pain in the hip, groin, or thigh, as this may be an early indication of a possible fracture of the thigh.
Rarely, allergic reactions may occur in patients receiving Osvyrti. Symptoms include swelling in the face, lips, tongue, throat, or other parts of the body; rash, itching, or hives on the skin; wheezing or difficulty breathing. Inform your doctorif you experience such symptoms while being treated with Osvyrti.
Very common side effects(may affect more than 1 in 10 people):
- bone, joint, and/or muscle pain, which can be intense,
- pain in the legs or arms (pain in the extremities).
Common side effects(may affect up to 1 in 10 people):
- painful urination, frequent urination, presence of blood in the urine, urinary incontinence,
- upper respiratory tract infection,
- pain, numbness, or tingling that extends to the lower leg (sciatica),
- constipation,
- abdominal discomfort,
- skin rash,
- skin condition with itching, redness, and/or dryness (eczema),
- hair loss (alopecia).
Uncommon side effects(may affect up to 1 in 100 people):
- fever, vomiting, and abdominal pain or discomfort (diverticulitis),
- ear infection,
- skin rash or mouth ulcers (drug-induced lichenoid eruptions).
Rare side effects(may affect up to 1 in 10,000 people):
- allergic reaction that can damage blood vessels, mainly in the skin (e.g., purple or reddish-brown spots, hives, or skin ulcers) (hypersensitivity vasculitis).
Frequency not known(cannot be estimated from the available data):
- consult your doctor if you have ear pain, discharge from the ear, and/or an ear infection. These could be symptoms of damage to the bones of the ear.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Osvyrti
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after "EXP". The expiry date refers to the last day of the month shown.
Store in a refrigerator (2°C to 8°C).
Do not freeze.
Keep the pre-filled syringe in the outer packaging to protect it from light.
Before injection, the pre-filled syringe can be left outside the refrigerator to reach room temperature (up to 25°C). This will make the injection less painful. Once the pre-filled syringe has reached room temperature (up to 25°C), it should be used within 30 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Osvyrti Composition
- The active ingredient is denosumab. Each 1 ml pre-filled syringe contains 60 mg of denosumab (60 mg/ml).
- The other components are glacial acetic acid, sodium hydroxide, sorbitol (E420), polysorbate 20, and water for injectable preparations (see section 2, Osvyrti contains sorbitol, Osvyrti contains polysorbate 20, and Osvyrti contains sodium).
Osvyrti Appearance and Container Contents
Osvyrti is a clear, colorless to pale yellow, injectable solution, available in a pre-filled syringe ready for use.
Each container contains a pre-filled syringe with a needle guard.
Only certain pack sizes may be marketed.
Marketing Authorization Holder
Accord Healthcare S.L.U.
World Trade Center, Moll de Barcelona, s/n
Edifici Est, 6a Planta
08039 Barcelona
Spain
Manufacturer
Accord Healthcare Polska Sp. z.o.o.
Ul. Lutomierska 50, 95-200,
Pabianice, Poland
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
AT / BE / BG / CY / CZ / DE / DK / EE / ES / FI / FR / HR / HU / IE / IS / IT / LT / LV / LU / MT / NL / NO / PL / PT / RO / SE / SI / SK
Accord Healthcare S.L.U.
Tel: +34 93 301 00 64
EL
Win Medica Α.Ε.
Τηλ: +30 210 74 88 821
Date of Last Revision of this Prospectus: <{MM/AAAA}><{month AAAA}>.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu
---------------------------------------------------------------------------------------------------------------------------------
Important |
Read this important information before using the Osvyrti pre-filled syringe with automatic needle guard:
XDo notremove the gray needle cap from the pre-filled syringe until you are ready for injection. XDo notuse the pre-filled syringe if it has been dropped onto a hard surface. Use a new pre-filled syringe and contact your doctor or healthcare professional. XDo notattempt to activate the pre-filled syringe before injection. ΧDo nottouch the activation clips of the needle guard before use, as the needle guard of the syringe will activate too early. XDo notattempt to remove the transparent safety guard from the pre-filled syringe. If you have any doubts, contact your doctor or healthcare professional. |
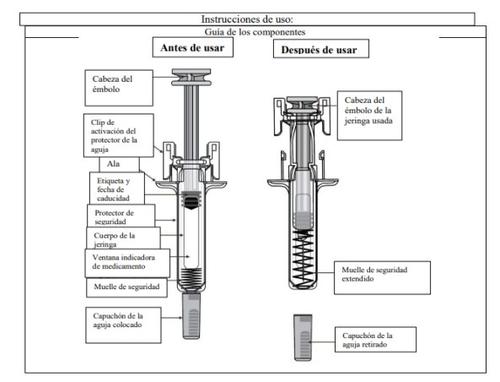
Step 1: Preparation | |
A | Remove the pre-filled syringe container from the packaging and gather the materials you need for your injection: alcohol swabs, cotton balls or gauze, a plaster, and a container for disposing of sharp objects (not included). |
To make the injection less painful, leave the pre-filled syringe at room temperature for about 30 minutes before the injection. Wash your hands carefully with soap and water. Place the new pre-filled syringe and other materials on a clean, well-lit surface. X Do notattempt to warm the syringe using a heat source such as hot water or a microwave. X Do notleave the pre-filled syringe exposed to direct sunlight. X Do notshake the pre-filled syringe.
|
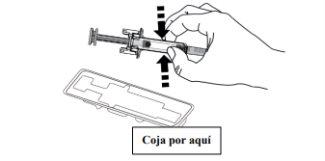
B | Open the container by removing the cover. Hold the pre-filled syringe by the safety guard to remove it from the container. |
For safety reasons: X Do nothold it by the plunger head X Do nothold it by the gray needle cap. |
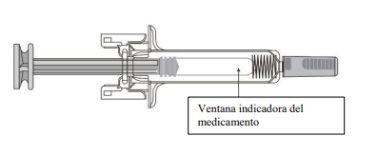
C | Inspect the medication and the pre-filled syringe. |
| |
XDo notuse the pre-filled syringe if:
In any of these cases, contact your doctor or healthcare professional. |
Step 2: Prepare | |
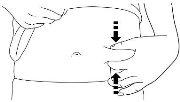
A | Wash your hands carefully. Prepare and clean the injection site. |
You can inject the medication into:
X Do nottouch the injection site before injecting. Do notinject into areas where the skin is sensitive, bruised, red, or hardened. Avoid injecting into areas with scars or stretch marks. |
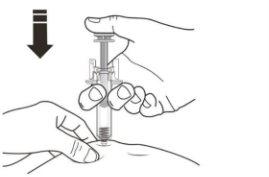
B | Carefully pull the gray needle cap straight off, keeping the syringe away from your body. |
Do nottwist or bend the needle cap. Do nothold the pre-filled syringe by the plunger. Dispose of the needle cap in the container for sharp objects. Do nottouch the needle or let it touch any surface. Do notput the cap back on the needle. |
C | Pinch the injection site to create a firm surface. |
It is essential to keep the skin pinched when injecting. |
Step 3: Inject | |
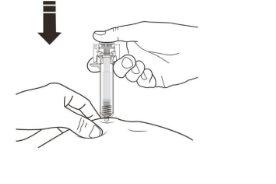
A | Keep the skin pinched. INSERT the needle into the skin. |
X Do nottouch the clean area of the skin. |
B | PRESS the plunger head with light, constant pressure until you feel or hear a "click". Push all the way down until you hear the "click". |
It is essential to press down until you hear the "click" to receive your full dose. |
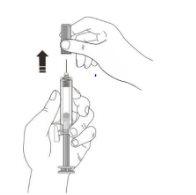
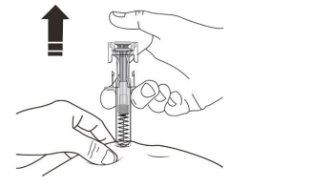
C | RELEASE the plunger head. Then, REMOVE the syringe from the skin. |
After releasing the plunger head, the safety guard of the pre-filled syringe will safely cover the needle. X Do notput the gray needle cap back on used pre-filled syringes. |
Step 4: Final | |
A | Dispose of the used pre-filled syringe and other materials in a container for sharp objects. |
Medicines should be disposed of in accordance with local regulations. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment. Keep the syringe and the container for sharp objects out of the sight and reach of children. X Do notreuse the pre-filled syringe. X Do notrecycle the pre-filled syringes or throw them in the trash. |
B | Examine the injection site. |
If you see blood, press the injection site with a cotton ball or gauze. Do notrub the injection site. If necessary, apply a plaster. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OSVYRTI 60 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 60 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription required
Online doctors for OSVYRTI 60 mg Injectable Solution in Pre-filled Syringe
Discuss questions about OSVYRTI 60 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions