OMNITROPE 5 mg/1.5 ml INJECTABLE SOLUTION IN CARTRIDGE

How to use OMNITROPE 5 mg/1.5 ml INJECTABLE SOLUTION IN CARTRIDGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
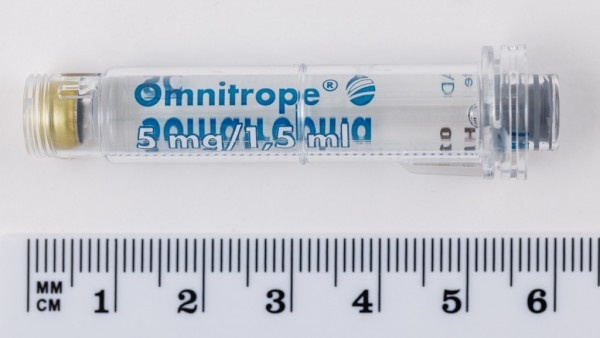
Omnitrope 5mg/1.5ml solution for injection
Omnitrope 10mg/1.5ml solution for injection
Somatropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Omnitrope is and what it is used for
- What you need to know before you use Omnitrope
- How to use Omnitrope
- Possible side effects
- Storage of Omnitrope
- Contents of the pack and other information
1. What Omnitrope is and what it is used for
Omnitrope is a recombinant human growth hormone (also called somatropin). It has the same structure as the natural human growth hormone, which is necessary for bones and muscles to grow. It also helps fat and muscle tissues to develop in the right amounts. It is recombinant, which means it is not made from human or animal tissue.
In children, Omnitrope is used to treat the following growth disorders:
- If you are not growing properly and do not have enough growth hormone of your own.
- If you have Turner syndrome, a genetic disorder in girls that can affect growth; your doctor will have told you if you have this disorder.
- If you have chronic renal failure. As your kidneys lose their ability to function normally, this can affect growth.
- If you were too small or too light at birth. Growth hormone can help you grow more if you have not had a growth spurt or maintained normal growth by the age of four or later.
- If you have Prader-Willi syndrome (a chromosomal disorder). Growth hormone can help you grow more if you are still growing and will also improve your body composition. Excess fat will decrease and reduced muscle mass will improve.
In adults, Omnitrope is used to
- treat people with a pronounced deficiency of growth hormone. This can start during adulthood or can continue from childhood.
If you have been treated with Omnitrope for a growth hormone deficiency during childhood, your growth hormone status will be re-examined after you have finished growing. If a severe deficiency is confirmed, your doctor will propose continuing treatment with Omnitrope.
You should only receive this medicine from a doctor who has experience with growth hormone and has confirmed your diagnosis.
2. What you need to know before you use Omnitrope
Do not use Omnitrope
- if you are allergic (hypersensitive) to somatropin or any of the other ingredients of Omnitrope.
- and tell your doctor if you have an active tumor (cancer). Tumors must be inactive and you must have finished your anti-tumor treatment before starting treatment with Omnitrope.
- and tell your doctor if you have been prescribed Omnitrope to stimulate growth but you have already stopped growing (epiphyses closed).
- if you are severely ill (for example, post-surgical complications, open-heart surgery, abdominal surgery, accidental trauma, acute respiratory failure, or similar conditions). If you are going to have or have had major surgery, or if you are going to the hospital for any reason, tell your doctor and remind the other doctors you see that you are using growth hormone.
Warnings and precautions
Consult your doctor before starting Omnitrope.
- If you are receiving glucocorticoid replacement therapy, you should consult your doctor regularly as it may be necessary to adjust your glucocorticoid dose.
- If you are at risk of developing diabetes, your doctor should regularly check your blood glucose levels during treatment with somatropin.
- If you have diabetes mellitus, you should closely monitor your blood glucose levels during treatment with somatropin and discuss the results with your doctor to decide if you need to change the dose of your diabetes medications.
- After starting treatment with somatropin, some patients may need to start thyroid hormone replacement.
- If you are receiving thyroid hormone treatment, it may be necessary to adjust the dose of thyroid hormone.
- If you have increased intracranial pressure (which causes symptoms such as severe headache, visual disturbances, or vomiting), you should inform your doctor.
- If you start limping or develop a limp during treatment with growth hormone, you should inform your doctor.
- If you are receiving somatropin for a growth hormone deficiency after a previous tumor (cancer), you should be regularly examined to detect any recurrence of the tumor or any other cancer.
- If you experience worsening abdominal pain, you should inform your doctor.
- Experience in patients over 80 years is limited. Elderly people may be more sensitive to the effects of somatropin and may therefore be more prone to adverse reactions.
Children with chronic renal failure
- Your doctor should examine your kidney function and growth rate before starting treatment with somatropin. Medical treatment for your kidneys should continue. Treatment with somatropin should be interrupted in case of kidney transplant.
Children with Prader-Willi syndrome
- Your doctor will give you dietary restrictions to follow to control your weight.
- Your doctor will assess the signs of upper airway obstruction, sleep apnea (where breathing stops during sleep), or respiratory infection before starting treatment with somatropin.
- During treatment with somatropin, inform your doctor if you develop signs of upper airway obstruction (even if you start snoring or your snoring worsens). Your doctor may need to examine you and may interrupt treatment with somatropin.
- During treatment, your doctor will examine you for signs of scoliosis, a type of spinal deformity.
- During treatment, if you develop a lung infection, inform your doctor so that they can treat the infection.
Children born too small or underweight
- If you were too small or too light at birth and are between 9 and 12 years old, consult your doctor specifically about puberty and treatment with this medicine.
- Treatment should continue until you have stopped growing.
- Your doctor will check your glucose and insulin levels before starting treatment and every year during treatment.
Using Omnitrope with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
In particular, tell your doctor if you are taking or have recently taken any of the following medicines. Your doctor may need to adjust the dose of Omnitrope or the other medicines:
- medicines for diabetes;
- thyroid hormones;
- medicines to control epilepsy (anticonvulsants);
- cyclosporin (a medicine that weakens the immune system after transplants);
- oral estrogens or other sex hormones;
- synthetic adrenal hormones (corticosteroids).
Your doctor may need to adjust the dose of these medicines or the dose of somatropin.
Pregnancy and breastfeeding
Do not use Omnitrope if you are pregnant or trying to become pregnant.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Important information about some of the ingredients of Omnitrope
This medicine contains less than 1 mmol of sodium (23 mg) per ml; it is essentially "sodium-free".
Omnitrope 5 mg/1.5 ml solution for injection:
1 ml contains 9 mg of benzyl alcohol.
Due to the presence of benzyl alcohol, the medicine should not be administered to premature or newborn children. It may cause toxic reactions and allergic reactions in children up to 3 years of age.
3. How to use Omnitrope
Follow the administration instructions for this medication exactly as indicated by your doctor, pharmacist, or nurse. In case of doubt, consult your doctor, pharmacist, or nurse again.
The dose depends on your size, the condition being treated, and how well the growth hormone works in you. All people are different. Your doctor will advise you about your individualized dose of Omnitrope in milligrams (mg) based on your body weight in kilograms (kg) or body surface area, calculated from your height and weight in square meters (m2), as well as your treatment regimen. Do not change the dosage and treatment regimen without consulting your doctor.
The recommended dose is for:
Children with growth hormone deficiency:
0.025 to 0.035 mg/kg body weight per day or 0.7 to 1.0 mg/m2 body surface area per day. Higher doses may be used. When growth hormone deficiency continues during adolescence, Omnitrope should be continued until physical development is complete.
Girls with Turner syndrome:
0.045 to 0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day.
Children with chronic renal insufficiency:
0.045 to 0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day. Higher doses may be necessary if the growth rate is too low. A dose adjustment may be necessary after six months of treatment.
Children with Prader-Willi syndrome:
0.035 mg/kg body weight per day or 1.0 mg/m2 body surface area per day. The daily dose should not exceed 2.7 mg. Treatment should not be used in children who have almost stopped growing after puberty.
Children born smaller or with lower weight than expected and with a growth disorder:
0.035 mg/kg body weight per day or 1.0 mg/m2 body surface area per day. It is essential to continue treatment until final height is reached. Treatment should be discontinued after the first year if there is no response, or if final height has been reached and growth has stopped.
Adults with growth hormone deficiency:
If you continue using Omnitrope after childhood treatment, you should start with 0.2 to 0.5 mg per day.
This dosage should be gradually increased or decreased according to blood test results, as well as clinical response and side effects.
If growth hormone deficiency begins during adult life, you should start with 0.15 to 0.3 mg per day. This dosage should be gradually increased according to blood test results, as well as clinical response and side effects. The daily maintenance dose is rarely more than 1.0 mg per day. Women may need higher doses than men. The dosage should be monitored every six months. People over 60 years old should start with a dose of 0.1 to 0.2 mg per day, which should be gradually increased according to individual needs. The minimum effective dose should be used. The maintenance dose rarely exceeds 0.5 mg per day. Follow the instructions given by your doctor.
Omnitrope injection
Inject the growth hormone at about the same time every day. Bedtime is a good time because it is easy to remember. Additionally, it is natural to have a higher concentration of growth hormone at night.
Omnitrope 5 mg/1.5 ml is intended for multiple uses. It should only be administered with the Omnitrope Pen 5, an injection device specifically developed for use with Omnitrope 5 mg/1.5 ml injectable solution.
Omnitrope 10 mg/1.5 ml is intended for multiple uses. It should only be administered with the Omnitrope Pen 10, an injection device specifically developed for use with Omnitrope 10 mg/1.5 ml injectable solution.
Omnitrope is indicated for subcutaneous use. This means it is injected through a small needle into the fatty tissue under the skin. Most people inject themselves in the thigh or buttocks. Put the injection in the site your doctor has taught you. The fatty tissue under the skin may become reduced at the injection site. To avoid this, use a slightly different site each time you inject yourself. This gives the skin and the area underneath time to recover from an injection before receiving another one in the same spot.
Your doctor should have already taught you how to use Omnitrope. Always inject Omnitrope as your doctor has told you. If you are not sure, check with your doctor or pharmacist.
How to inject Omnitrope
The following instructions explain how to inject Omnitrope yourself. Read the instructions carefully and follow them step by step. Your doctor or nurse will teach you how to inject Omnitrope. Do not attempt to inject yourself unless you are sure you understand the procedure and what the injection involves.
- Omnitrope is administered as an injection under the skin.
- Inspect the solution carefully before injecting it and use it only if it is clear and colorless.
- Change the injection site to minimize the risk of local lipodystrophy (local reduction of fatty tissue under the skin).
Preparation | |
Before starting, you should have everything you need: |
|
| |
| |
| |
| |
Wash your hands before continuing with the following steps. | |
Omnitrope injection | |
|
|
| |
| |
| |
|
|
| |
After injecting | |
| |
| |
| |
|
If you use more Omnitrope than you should
If you inject much more than you should, consult your doctor or pharmacist as soon as possible. Your blood sugar levels may drop too low and then rise too high. You may feel shaky, sweaty, sleepy, or "not yourself," and you may pass out.
If you forget to use Omnitrope
Do not use a double dose to make up for forgotten doses. It is best to use the growth hormone regularly. If you forget to use a dose, take the next injection at the usual time the next day. Take note of the forgotten injections and inform your doctor at the next check-up.
If you stop treatment with Omnitrope
Consult your doctor before stopping the use of Omnitrope.
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone gets them. Very common and common side effects in adults may start in the first few months of treatment and may stop spontaneously or if the dose is reduced.
Very common side effects (which may affect more than 1 in 10 patients) include:
In adults
- Joint pain
- Water retention (which appears as swelling of the fingers or ankles)
Common side effects (which may affect less than 1 in 10 patients) include:
In children
- Redness, itching, or temporary pain at the injection site
- Joint pain
In adults
- Numbness, tingling
- Stiffness of the arms and legs, muscle pain
- Pain or tingling sensation in the hands or forearms (known as carpal tunnel syndrome)
Uncommon side effects (which may affect less than 1 in 100 patients) include:
In children
- Water retention (which appears as swelling of the fingers or ankles, for a short time at the start of treatment)
Rare side effects (which may affect less than 1 in 1,000 patients) include:
In children
- Numbness, tingling
- Leukemia (has been observed in a small number of patients with growth hormone deficiency, some of whom had been treated with somatropin. However, there is no indication that the incidence of leukemia is higher in growth hormone recipients without predisposing factors)
- Increased intracranial pressure (which causes symptoms such as severe headache, vision problems, or vomiting)
- Muscle pain
Frequency not known: cannot be estimated from the available data
- Type 2 diabetes
- Decreased cortisol hormone levels in the blood
In children
- Stiffness in the arms and legs
In adults
- Increased intracranial pressure (which causes symptoms such as severe headache, vision problems, or vomiting)
- Redness, itching, or pain at the injection site
Formation of antibodies against the injected growth hormone, but these do not seem to make the growth hormone stop working.
The skin around the injection site may become irregular or lumpy, but this should not happen if you inject in a different place each time.
There have been rare cases of sudden death in patients with Prader-Willi syndrome. However, these cases have not been related to treatment with Omnitrope.
Your doctor may consider a slipped capital femoral epiphysis or Legg-Calvé-Perthes disease if you experience discomfort or pain in the hip or knee while being treated with Omnitrope.
Other possible side effects related to your treatment with growth hormone may include:
You (or your child) may have high blood sugar levels or low thyroid hormone levels. Your doctor can check this and, if necessary, prescribe the appropriate treatment. In rare cases, pancreatitis has been observed in patients treated with growth hormone.
If you think any of the side effects you are experiencing is serious, or if you notice any side effect not mentioned in this leaflet, tell your doctor or pharmacist.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the national reporting system included in Annex V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Omnitrope
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the label and carton after EXP. The expiration date is the last day of the month indicated.
- Store and transport refrigerated (between 2°C and 8°C).
- Do not freeze.
- Store in the original packaging to protect it from light.
- After the first injection, the cartridge should remain in the pen and should be stored in a refrigerator at a temperature of 2 to 8 °C and should only be used for a maximum of 28 days.
Do not use Omnitrope if you notice that the solution is cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Composition of Omnitrope 5 mg/1.5 ml
- The active ingredient of Omnitrope is somatropin.
Each ml of solution contains 3.3 mg of somatropin (which corresponds to 10 IU).
A cartridge contains 5.0 mg (which corresponds to 15 IU) of somatropin in 1.5 ml.
- The other ingredients are:
Disodium phosphate heptahydrate
Sodium dihydrogen phosphate dihydrate
Mannitol
Poloxamer 188
Benzyl alcohol
Water for injectable preparations
Composition of Omnitrope 10 mg/1.5 ml
- The active ingredient of Omnitrope is somatropin.
Each ml of solution contains 6.7 mg of somatropin (which corresponds to 20 IU).
A cartridge contains 10.0 mg (which corresponds to 30 IU) of somatropin in 1.5 ml.
- The other ingredients are:
Disodium phosphate heptahydrate
Sodium dihydrogen phosphate dihydrate
Glycine
Poloxamer 188
Phenol
Water for injectable preparations
Appearance and packaging of the product
Omnitrope is a clear and colorless injectable solution.
Packages with 1, 5, or 10 cartridges.
Not all pack sizes may be marketed.
Marketing authorization holder
Sandoz GmbH
Biochemiestr. 10
A-6250 Kundl
Austria
Manufacturer
Sandoz GmbH
Biochemiestr. 10
A-6336 Langkampfen
Austria
Date of last revision of this leaflet: {MM/YYYY}
Detailed information on this medication is available on the European Medicines Agency website http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OMNITROPE 5 mg/1.5 ml INJECTABLE SOLUTION IN CARTRIDGEDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 0.2 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for OMNITROPE 5 mg/1.5 ml INJECTABLE SOLUTION IN CARTRIDGE
Discuss questions about OMNITROPE 5 mg/1.5 ml INJECTABLE SOLUTION IN CARTRIDGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions