OBODENCE 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use OBODENCE 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Obodence 60 mg solution for injection in pre-filled syringe
denosumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- Your doctor will provide you with a patient reminder card, which contains important safety information that you should know before and during treatment with Obodence.
Contents of the pack
- What is Obodence and what is it used for
- What you need to know before you use Obodence
- How to use Obodence
- Possible side effects
- Storage of Obodence
- Contents of the pack and other information
1. What is Obodence and what is it used for
What is Obodence and how does it work
Obodence contains denosumab, a protein (monoclonal antibody) that interferes with the action of another protein to treat bone loss and osteoporosis. Treatment with Obodence strengthens bones and reduces the risk of fractures.
Bone is a living tissue that is constantly renewed. Estrogens contribute to the preservation of bone health. After menopause, estrogen levels decrease, which can cause bones to become thinner and more fragile. Over time, this can lead to a disease called osteoporosis. Osteoporosis can also occur in men due to various causes, including age and/or low levels of the male hormone, testosterone. It can also occur in patients undergoing treatment with glucocorticoids. Many patients with osteoporosis do not have symptoms, although they still have a risk of fracturing bones, especially in the spine, hip, and wrists.
Surgical interventions or medications that stop the production of estrogen or testosterone, used to treat patients with prostate or breast cancer, can also cause bone loss. This makes bones weaker and more prone to breaking.
What is Obodence used for
Obodence is used to treat:
- postmenopausal osteoporosis in women and men who have an increased risk of fracture (bone break), reducing the risk of fractures of the hip, spine, and non-spinal locations.
- bone loss caused by reduced hormonal levels (testosterone) as a result of surgical intervention or treatment with medications in patients with prostate cancer.
- bone loss resulting from long-term treatment with glucocorticoids in patients who have a high risk of fracture.
2. What you need to know before you use Obodence
Do not use Obodence
- if you have low levels of calcium in the blood (hypocalcemia).
- if you are allergic to denosumab or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Obodence.
During treatment with Obodence, you may develop a skin infection with symptoms such as an inflamed and reddened area on the skin, more frequently on the lower leg, which feels hot and sensitive to the touch (cellulitis), and may be accompanied by fever. Inform your doctor immediately if you experience any of these symptoms.
In addition, you should take calcium and vitamin D supplements during treatment with Obodence. Your doctor will discuss this with you.
While receiving Obodence, you may experience low levels of calcium in the blood. Inform your doctor immediately if you notice any of the following symptoms: muscle spasms, contractions, or cramps, and/or numbness or tingling in the fingers of the hands, feet, or around the mouth, and/or convulsions, confusion, or loss of consciousness.
Rarely, cases of very low levels of calcium in the blood have been reported, which have required hospitalization and, in some cases, potentially life-threatening reactions. Therefore, before each dose is administered and, in patients with a predisposition to hypocalcemia, within two weeks after the initial dose, your blood calcium levels will be checked (through a blood test).
Inform your doctor if you have or have had severe kidney problems, renal failure, if you have needed to undergo dialysis, or if you are taking medications called glucocorticoids (such as prednisolone or dexamethasone), as they may increase the risk of having low levels of calcium in the blood if you do not take calcium supplements.
Problems in the mouth, teeth, or jaw
In patients receiving Obodence for osteoporosis, a rare side effect called osteonecrosis of the jaw (ONJ) (damage to the jawbone) has been reported (may affect up to 1 in 1,000 people). The risk of ONJ increases in patients treated for a long time (may affect up to 1 in 200 people if treated for 10 years). ONJ can also occur after stopping treatment. It is essential to try to prevent the development of ONJ, as it can be a painful condition that can be difficult to treat. To reduce the risk of developing ONJ, follow these precautions:
Before receiving treatment, inform your doctor or nurse (healthcare professional) if:
- you have any problems in your mouth or teeth, such as poor dental health, gum disease, or a planned tooth extraction.
- you do not receive regular dental check-ups or have not had a dental check-up for a long time.
- you are a smoker (as this may increase the risk of dental problems).
- you have been previously treated with a bisphosphonate (used to prevent or treat bone disorders).
- you are taking medications called corticosteroids (such as prednisolone or dexamethasone).
- you have cancer.
Your doctor may ask you to undergo a dental check-up before starting treatment with Obodence.
During treatment, you should maintain good oral hygiene and undergo routine dental check-ups. If you use dental prosthetics, ensure they fit properly. If you are undergoing dental treatment or are about to undergo dental surgery (e.g., tooth extractions), inform your doctor about your dental treatment and inform your dentist that you are being treated with Obodence.
Contact your doctor and dentist immediately if you experience any problems in your mouth or teeth, such as loose teeth, pain or inflammation, or ulcers that do not heal or are suppurating, as these could be symptoms of ONJ.
Unusual fractures of the thigh
Some people have developed unusual fractures in the thigh while being treated with denosumab. Consult your doctor if you experience new or unusual pain in the hip, groin, or thigh.
Children and adolescents
Obodence should not be used in children and adolescents under 18 years of age.
Other medicines and Obodence
Inform your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. It is especially important that you inform your doctor if you are being treated with another medicine that contains denosumab.
Do not use Obodence with another medicine that contains denosumab.
Pregnancy and breastfeeding
Denosumab has not been tested in pregnant women. It is essential that you inform your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant. Obodence is not recommended during pregnancy. Women of childbearing age should use effective contraceptive methods during treatment with Obodence and for at least 5 months after stopping treatment with Obodence.
If you become pregnant during treatment with Obodence or less than 5 months after stopping treatment with Obodence, inform your doctor.
It is not known whether denosumab is excreted in breast milk. It is essential that you inform your doctor if you are breastfeeding or plan to breastfeed. Your doctor will help you decide whether to stop breastfeeding or stop Obodence, considering the benefit of breastfeeding for the child and the benefit of Obodence for the mother.
If you are breastfeeding during treatment with Obodence, please inform your doctor.
Consult your doctor or pharmacist before using any other medicine.
Driving and using machines
Obodence has no or negligible influence on the ability to drive and use machines.
Sorbitol (E 420)
This medicine contains 44 mg of sorbitol (E 420) per ml of solution.
Sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 60 mg; this is essentially "sodium-free".
3. How to use Obodence
The recommended dose is a single pre-filled syringe of 60 mg administered under the skin (subcutaneously) once every 6 months. The best places for injection are the upper thighs and abdomen. If the injection is given by a caregiver (the person taking care of you), it can also be administered in the outer aspect of the upper arm. Consult your doctor for the date of the next possible injection.
In addition, you should take calcium and vitamin D supplements during treatment with Obodence. Your doctor will discuss this with you.
Your doctor may decide whether it is better for you or a caregiver to administer the Obodence injection. Your doctor or healthcare professional will show you or your caregiver how to use Obodence. If you want to obtain instructions on how to inject Obodence, read the last section of this leaflet.
Do not shake.
If you miss a dose of Obodence
If you miss a dose of Obodence, the injection should be administered as soon as possible. Subsequent injections should be scheduled every 6 months from the date of the last injection.
If you stop treatment with Obodence
To get the most benefit from your treatment and reduce the risk of fractures, it is essential that you use Obodence for the entire period prescribed by your doctor. Do not stop treatment without talking to your doctor first.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Patients treated with Obodence may develop skin infections (mainly cellulitis) infrequently. Tell your doctor immediatelyif you experience any of these symptoms during treatment with Obodence: a swollen and reddened area on the skin, usually on the lower leg, which feels hot and sensitive to the touch, and may be accompanied by fever.
Rarely, patients receiving Obodence may develop pain in the mouth and/or jaw, inflammation, or ulcers that do not heal in the mouth or jaw, suppurating, numbness, or a feeling of heaviness in the jaw, or tooth mobility. These could be symptoms of bone damage in the jaw (osteonecrosis). Tell your doctor and dentist immediatelyif you experience such symptoms while being treated with Obodence or after stopping treatment.
Rarely, patients receiving Obodence may experience low levels of calcium in the blood (hypocalcemia); very low levels of calcium in the blood can require hospitalization and, in some cases, potentially life-threatening reactions. Symptoms include muscle spasms, contractions, or cramps, and/or numbness or tingling in the fingers of the hands, feet, or around the mouth, and/or convulsions, confusion, or loss of consciousness. If you experience any of these, tell your doctor immediately. Low levels of calcium in the blood can also cause a change in heart rhythm called QT prolongation, which can be observed by performing an electrocardiogram (ECG).
Rarely, unusual fractures of the thigh can occur in patients receiving Obodence. Consult your doctorif you experience new or unusual pain in the hip, groin, or thigh, as this may be an early indication of a possible fracture of the thigh.
Rarely, allergic reactions can occur in patients receiving Obodence. Symptoms include swelling in the face, lips, tongue, throat, or other parts of the body; rash, itching, or hives on the skin; wheezing or difficulty breathing. Tell your doctorif you experience such symptoms while being treated with Obodence.
Very common side effects(may affect more than 1 in 10 people):
- bone, joint, and/or muscle pain, which can sometimes be severe,
- leg or arm pain (pain in the limbs).
Common side effects(may affect up to 1 in 10 people):
- painful urination, frequent urination, presence of blood in the urine, urinary incontinence,
- upper respiratory tract infection,
- pain, numbness, or tingling that extends to the lower leg (sciatica),
- constipation,
- abdominal discomfort,
- skin rash,
- skin condition with itching, redness, and/or dryness (eczema),
- hair loss (alopecia).
Uncommon side effects(may affect up to 1 in 100 people):
- fever, vomiting, and abdominal pain or discomfort (diverticulitis),
- ear infection,
- skin rash or mouth ulcers (drug-induced lichenoid eruptions).
Rare side effects(may affect up to 1 in 10,000 people):
- allergic reaction that can damage blood vessels, mainly in the skin (e.g., purple or reddish-brown spots, hives, or skin ulcers) (hypersensitivity vasculitis).
Frequency not known(cannot be estimated from the available data):
- consult your doctor if you have ear pain, your ear is suppurating, and/or you have an ear infection. These could be symptoms of damage to the bones of the ear.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Obodence
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Keep the pre-filled syringe in the outer packaging to protect it from light.
Before injection, the pre-filled syringe can be left at room temperature (up to 25°C) to allow it to reach room temperature. This will make the injection less painful.
Once the pre-filled syringe has reached room temperature (up to 25°C), it can be stored at room temperature for a single period of up to 60 days, without exceeding the original expiry date. If it is not used during this period of up to 60 days, the syringe can be returned to the refrigerator for 3 days to be used in the future. Do not use the syringe after the expiry date printed on the label.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Obodence
- The active ingredient is denosumab. Each 1 ml pre-filled syringe contains 60 mg of denosumab (60 mg/ml).
- The other components are histidine, histidine hydrochloride monohydrate, sorbitol (E 420), polysorbate 20, water for injectable preparations.
Appearance of the Product and Container Contents
Obodence is a clear, colorless to slightly yellowish injectable solution, available in a pre-filled syringe ready for use.
Each container contains a pre-filled syringe with a needle shield.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Samsung Bioepis NL B.V.
Olof Palmestraat 10
2616 LR Delft
Netherlands
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
Instructions for Use: |
Component Guide | |
Before Use | After Use |
|
|
Important |
Read this important information before using the Obodence pre-filled syringe with needle shield:
If you have any doubts, contact your doctor or healthcare professional. |
Step 1: Preparation | |
A | Hold the safety shield of the pre-filled syringe to remove it from the container and gather the materials you need for your injection: alcohol swabs, cotton balls or gauze, a plaster, and a sharps container (not included). |
For safety reasons:
To make the injection less painful, leave the pre-filled syringe at room temperature for about 30 minutes before injection. Wash your hands carefully with soap and water. Place the new pre-filled syringe and other materials on a clean, well-lit surface.
|
B | Examine the medicine and the pre-filled syringe. |
| |
Do notuse the pre-filled syringe if:
In any of these cases, contact your doctor or healthcare professional. |
Step 2: Prepare | |
A | Wash your hands carefully. Prepare and clean the injection site. |
You can inject the medicine into:
Clean the injection site with an alcohol swab. Let the skin dry.
|
B | Carefully pull the needle cap straight off, keeping the syringe away from your body. |
|
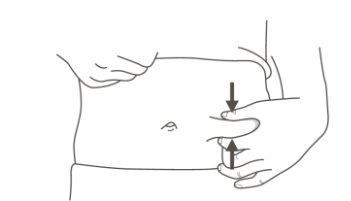
C | Pinch the injection site to create a firm surface. |
It is important to keep the skin pinched when injecting. |
Step 3: Inject | |
A | Keep the skin pinched. INSERT the needle into the skin. |
|
B | PRESS the plunger head with light and constant pressure until you feel or hear a "click". Push all the way down until you hear the "click". |
|
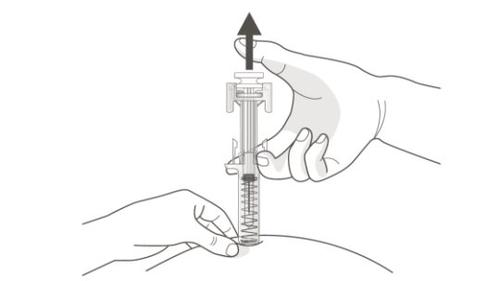
C | STOP PRESSING the plunger head. Then, REMOVE the syringe from the skin. |
After releasing the plunger head, the safety shield of the pre-filled syringe will safely cover the needle.
|
Step 4: Final | |
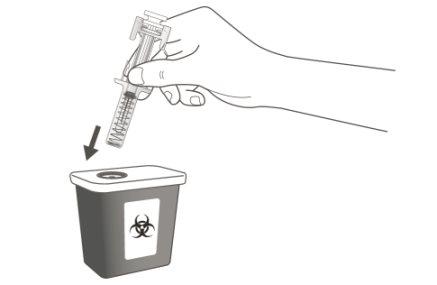
A | Discard the used pre-filled syringe and other materials in a sharps container. |
Medicines should be disposed of in accordance with local regulations. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment. Keep the syringe and sharps container out of sight and reach of children.
|
B | Examine the injection site. |
If you see blood, press the injection site with a cotton ball or gauze. Do not rub the injection site. If necessary, apply a plaster. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OBODENCE 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 120 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription requiredDosage form: INJECTABLE, 60 mgActive substance: denosumabManufacturer: Fresenius Kabi Deutschland GmbhPrescription required
Online doctors for OBODENCE 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about OBODENCE 60 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





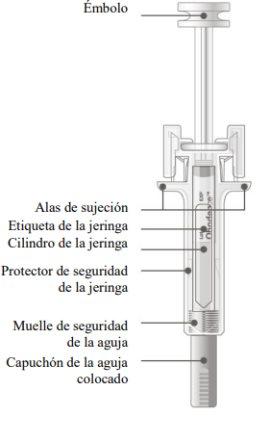
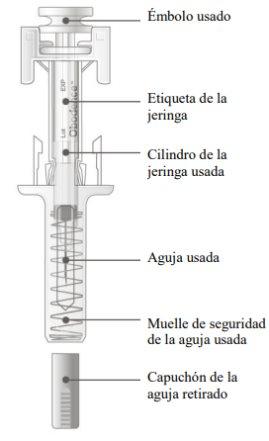
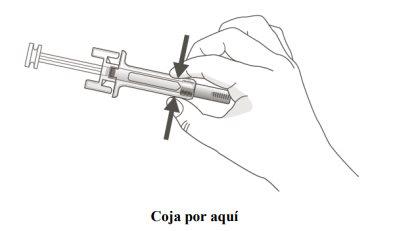
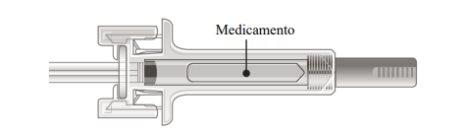
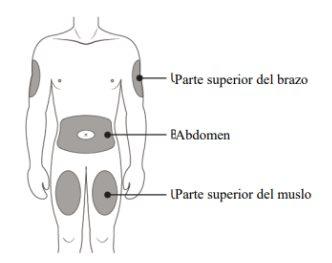

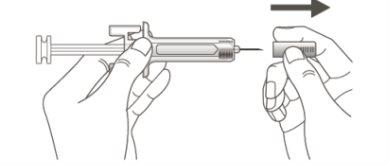
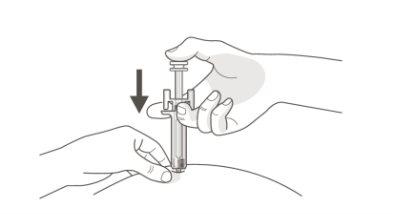
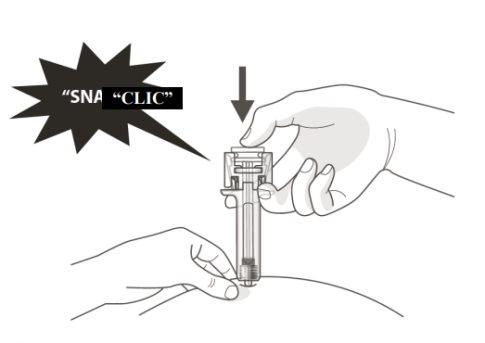
 It is important to press down until you hear the "click" to receive your full dose.
It is important to press down until you hear the "click" to receive your full dose.