
NUTROPINAQ 10 mg/2 ml (30 IU) INJECTABLE SOLUTION


How to use NUTROPINAQ 10 mg/2 ml (30 IU) INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
NutropinAq 10mg/2ml (30UI), injectable solution
Somatropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is NutropinAq and what is it used for
- What you need to know before you use NutropinAq
- How to use NutropinAq
- Possible side effects
- Storage of NutropinAq
- Contents of the pack and further information
1. What is NutropinAq and what is it used for
NutropinAq contains somatropin, which is a recombinant growth hormone similar to the natural human growth hormone produced by your body. It is recombinant, meaning it is made outside the body by a special process. Growth hormone (GH) is a chemical messenger produced by a small gland in your brain called the pituitary gland. In children, it tells the body to grow, helps bones develop normally, and later in adulthood, GH helps maintain normal body shape and metabolism.
In children, NutropinAq is used:
- When your body does not produce enough growth hormone and, as a result, is not growing properly.
- When they have Turner syndrome. Turner syndrome is a genetic anomaly in girls (absence of female sex chromosome(s)) that prevents growth.
- When the kidneys are damaged and lose their ability to function normally, affecting growth.
In adults, NutropinAq is used:
- If your body does not produce enough growth hormone as an adult. This can start during adult life or continue from childhood.
Benefits of using this medicine
In children, it helps the body and bones develop normally.
In adults, it helps maintain normal body shape and metabolism, for example, lipid profile and glucose levels.
2. What you need to know before you use NutropinAq
Do not use NutropinAq:
- if you are allergic to somatropin or any of the other ingredients of this medicine (listed in section 6).
- in children, if the bones have already stopped growing.
- if you have an active tumor (cancer). Tell your doctor if you have or have had an active tumor. Tumors must be inactive and you must have finished your anti-tumor treatment before starting treatment with NutropinAq.
- If you have had complications after major surgery (abdominal or open-heart surgery), multiple trauma, acute respiratory failure, or similar conditions.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with NutropinAq.
- If you experience visual changes, severe or frequent headaches, associated with a feeling of being unwell (nausea) or vomiting, especially at the start of treatment, contact your doctor immediately. These may be signs of a temporary increase in pressure in the brain (intracranial hypertension).
- If, during growth, you experience limping or pain in the hip or knee, consult your doctor.
- If you have a curvature of the spine (scoliosis), it is necessary for you to visit a doctor frequently, as scoliosis can progress in children during rapid growth.
- Your doctor should monitor your high sugar levels (hyperglycemia) during treatment with NutropinAq. If you are being treated with insulin, your doctor may need to adjust your insulin dose. If you have diabetes and severe or worsening eye disease, you should not be treated with NutropinAq.
- Your doctor should check your thyroid function periodically and, if necessary, prescribe appropriate treatment. If you have an underactive thyroid gland that produces low levels of thyroid hormone (hypothyroidism), you should be treated before starting therapy with NutropinAq. If your hypothyroidism is not treated, it may prevent NutropinAq from working.
- If you are receiving glucocorticoid replacement therapy, you should consult your doctor regularly, as it may be necessary to adjust your glucocorticoid dose.
- If you have had a tumor (cancer) in the past, especially a tumor that affects the brain, your doctor should pay special attention and examine you regularly for a possible recurrence of the tumor.
- A small number of patients with growth hormone deficiency have developed leukemia (blood cancer). However, no cause-and-effect relationship has been demonstrated with growth hormone treatment.
- If you undergo a kidney transplant, you should stop treatment with NutropinAq.
- If you experience complications after major surgery (abdominal or open-heart surgery), multiple trauma, acute respiratory failure, or similar conditions, your doctor should decide whether it is safe to continue treatment with NutropinAq.
- There may be an increased risk of developing pancreatitis in children compared to adults treated with growth hormone. In case of severe and persistent abdominal pain, consult your doctor.
- If you have Prader-Willi syndrome, you should not be treated with NutropinAq unless you have growth hormone deficiency.
Other medicines and NutropinAq
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
- If you are being treated with glucocorticoid replacement therapy, the effect of NutropinAq on growth may be reduced. You should consult your doctor regularly, as it may be necessary to adjust your glucocorticoid dose.
- If you are being treated with insulin, your doctor may adjust your insulin dose.
- If you are being treated with sex steroids, anticonvulsants, or cyclosporine, consult your doctor. If you have been diagnosed with adrenal insufficiency during treatment with NutropinAq, you need treatment with steroids. If you are already being treated for adrenal insufficiency, you may need an adjustment of your steroid dose.
- In particular, tell your doctor if you are taking or have recently taken any of the following medicines. Your doctor may need to adjust the dose of NutropinAq or the other medicines:
- Oral estrogens or other sex hormones
Pregnancy and breastfeeding
If you are pregnant, you should stop treatment with NutropinAq.
You should be cautious when breastfeeding during treatment with NutropinAq.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
No effects on the ability to drive or use machines have been observed while using NutropinAq.
NutropinAq is essentially “sodium-free”
This medicine contains less than 1mmol of sodium (23 mg) per vial, i.e., it is essentially sodium-free.
3. How to use NutropinAq
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Treatment with NutropinAq should be carried out under the regular supervision of a doctor with experience in growth hormone deficiency.
The dose of NutropinAq to be injected will be decided by your doctor. Do not change the dose without consulting your doctor. The recommended dose is:
In children with growth hormone deficiency:
0.025-0.035 mg/kg body weight injected every day under the skin (subcutaneous injection).
In girls with Turner syndrome:
Up to 0.05 mg/kg body weight injected every day under the skin (subcutaneous injection).
In children with chronic renal insufficiency:
Up to 0.05 mg/kg body weight injected every day under the skin (subcutaneous injection). Treatment with NutropinAq can continue until the time of kidney transplantation.
In adults with growth hormone deficiency:
Low initial doses of 0.15-0.3 mg injected every day under the skin (subcutaneous injection). Your doctor may then increase the dose depending on your response. The final dose rarely exceeds 1.0 mg/day. In general, the minimum dose that produces a response should be administered.
Treatment with NutropinAq is long-term therapy. For more information, consult your doctor.
How to inject NutropinAq
The dose of NutropinAq to be injected will be decided by your doctor. You must inject NutropinAq every day under the skin (subcutaneous injection). It is important to change the injection site every day to avoid damaging the skin.
NutropinAq is supplied in a multi-dose solution. After removal from the refrigerator, if the solution is cloudy, the contents should not be injected. Shake gently. Do not shake vigorously, as this may denature the protein.
To inject NutropinAq, you must use the NutropinAq Pen. For each injection, you must use a new sterile injection needle. Read all the instructions for use (on the back of this leaflet) carefully before starting to use the NutropinAq Pen. At the start of treatment, it is recommended that a doctor or nurse administer the injection and teach you how to use the preloaded NutropinAq Pen. After this training, you will be able to inject yourself or your caregiver will be able to inject you.
If you use more NutropinAq than you should
If you have injected more NutropinAq than you should, consult your doctor. If you have injected too much NutropinAq, your blood sugar levels may decrease and then increase too much (hyperglycemia).
If you inject too much NutropinAq over a prolonged period (years), you may experience excessive growth of parts of your body, such as the ears, nose, lips, tongue, and cheekbones (gigantism and/or acromegaly).
If you forget to use NutropinAq
Do not take a double dose to make up for forgotten doses. The next day, continue with your usual dose and tell your doctor at your next visit.
If you stop treatment with NutropinAq
Consult your doctor before stopping treatment with NutropinAq. If you stop treatment too early or too late, the results will not be as expected.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Consult your doctor immediately if you notice any change or increase in the growth of birthmarks and/or freckles (melanocytic nevi). In case of tumor or recurrence of previous tumors (confirmed by your doctor), treatment with NutropinAq should be discontinued immediately. This adverse effect is rare, it can affect 1 in 100 patients.
Consult your doctor immediately if you experience visual changes, severe or frequent headaches, associated with discomfort (nausea) or vomiting. These symptoms could be a temporary increase in pressure in the brain (intracranial hypertension). If you experience intracranial hypertension, your doctor may temporarily reduce or discontinue therapy with NutropinAq. Subsequently, therapy can start again when the episode has ended. These adverse effects are rare, they can affect 1 in 1,000 patients.
Other adverse effects include:
Very common (may affect more than 1 in 10 patients)
Swelling of the hands and feet due to fluid accumulation (peripheral edema), sometimes associated with muscle pain (myalgia) and joint pain (arthralgia). These adverse effects seem to be more common in adults and are short-lived. Edema has been reported as frequent in children.
Common (may affect 1 in 10 patients)
Underactivity of the thyroid gland resulting in low levels of thyroid hormones (hypothyroidism). If your hypothyroidism is not treated, it may cause NutropinAq to stop working. Your doctor should periodically monitor your thyroid function and, if necessary, prescribe appropriate treatment.
Reduced ability of your body to absorb sugar (glucose) from the blood, generating high blood sugar levels (hyperglycemia). Your doctor should monitor you to rule out possible signs of this effect during treatment with NutropinAq. If you are being treated with insulin, it may be necessary for your doctor to adjust your insulin dose.
Feeling of weakness (asthenia) and increased muscle tension (hypertonia).
Pain, bleeding, bruising (bruises), rashes, and itching at the injection site. These effects can be avoided by using a correct injection technique and changing the injection points.
Some patients may develop antibodies (a type of protein produced by the body) against somatropin. When these antibodies are present in patients, they do not prevent them from continuing to grow.
Uncommon (may affect 1 in 100 people)
Decrease in the number of red blood cells in the blood (anemia), decrease in blood sugar levels (hypoglycemia), and increase in phosphate levels (hyperphosphatemia).
Changes in personality or abnormal behavior.
Persistent tingling, burning sensation, pain, and/or numbness of the palm of the hand due to pinching of the wrist nerve (carpal tunnel syndrome).
Involuntary eye movements (nystagmus), inflammation of the optic nerve in the eye (papilledema), double vision (diplopia), headache (cephalalgia), drowsiness, and vertigo.
Increased heart rate (tachycardia) and high blood pressure (hypertension).
Vomiting, stomach pain, gas (flatulence), and feeling of discomfort (nausea).
Dry and sensitive skin (exfoliative dermatitis), changes in skin thickness, excessive hair growth on the face and body (hirsutism), hives (urticaria).
Curvature of the spine (scoliosis). If you have scoliosis, you will need to be frequently monitored to check if there is an increase in curvature.
Alteration of bones such as the separation of the upper part of the leg (femur) from the hip (slipped capital femoral epiphysis). This usually occurs in patients who grow rapidly. Patients with endocrine disorders are more likely to develop slipped capital femoral epiphysis.
Muscle size reduction (muscle atrophy), joint pain (arthralgia), and bone pain.
Difficulty retaining urine (urinary incontinence), increased frequency (pollakiuria), and volume (polyuria).
Uterine bleeding (metrorrhagia) and genital discharge.
Localized fat gain/loss of skin (lipoatrophy, atrophy/hypertrophy at the injection site)
Increased adenoid size with symptoms similar to tonsil enlargement (see rare).
Rare (may affect 1 in 1,000 patients)
Increased blood sugar levels (hyperglycemia, diabetes mellitus). Diabetes mellitus can cause increased frequency of urination, thirst, and hunger. If you experience any of these symptoms, you should inform your doctor.
Enlargement of the tonsils that causes snoring, difficulty breathing or swallowing, interruption of breathing during sleep (sleep apnea), or fluid in the ears, as well as ear infections. If this is particularly bothersome, you should discuss it with your doctor.
Abnormal sensation of tingling, pinching, and numbness (paresthesia), abnormal bone development, disease that affects the progress of bone growth (osteochondritis), and muscle weakness.
Other rare adverse effects of treatment with NutropinAq include itching all over the body, rash, blurred vision, weight gain, dizziness, diarrhea, facial swelling, fatigue, pain, fever, breast enlargement (gynecomastia), depression, and difficulty sleeping (insomnia).
Adverse Effects Specific to Indication, Observed in Clinical Trials
In children with growth hormone deficiency, brain tumors (central nervous system) have been frequently reported. Of the 236 patients included in the clinical trials, 3 patients presented with a central nervous system tumor, 2 patients experienced a recurrent medulloblastoma, and 1 patient experienced a histiocytoma. See also the "Warnings and Precautions" section.
In girls with Turner syndrome, abnormally heavy menstrual bleeding has been frequently reported.
In children with chronic renal failure, peritonitis, bone necrosis, and increased creatinine levels in the blood have been frequently reported. They are more likely to develop increased pressure in the brain (intracranial hypertension), with a higher risk at the start of treatment, although children with growth hormone deficiency and Turner syndrome also have an increased incidence.
In adults with growth hormone deficiency, paresthesia, high blood glucose levels, high lipid levels in the blood, insomnia, joint disorders, arthrosis (degenerative joint disease), muscle weakness, back pain, breast pain, and breast enlargement (gynecomastia) have been frequently reported.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Annex V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of NutropinAq
Keep out of sight and reach of children.
Do not use this medicine after the expiration date stated on the cartridge label after CAD. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the blister in the outer packaging.
After first use, the cartridge can be stored for up to 28 days between 2°C-8°C. Do not remove the cartridge in use from the NutropinAq Pen pre-filled pen between injections.
Do not use NutropinAq if you notice that the solution has become cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
What does NutropinAq contain?
- The active substance of NutropinAq is somatropin*.
- Somatropin is a human growth hormone produced in Escherichia coli cells by recombinant DNA technology.
- The other ingredients (excipients) are sodium chloride, phenol, polysorbate 20, sodium citrate dihydrate, anhydrous citric acid, and water for injectable preparations.
Appearance of the Product and Package Contents
NutropinAq is an injectable solution (in a cartridge (10 mg/2 ml) - packages of 1, 3, and 6). The solution for multiple doses is clear and colorless.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Ipsen Pharma, 65 quai Georges Gorse, 92100 Boulogne-Billancourt, France
Manufacturer:
IPSEN PHARMA BIOTECH S.A.S., Parc d’Activités du Plateau de Signes, CD no 402, 83870 Signes, France
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder.
België/Belgique/Belgien, Luxembourg/Luxemburg Ipsen NV Guldensporenpark 87 B-9820 Merelbeke België /Belgique/Belgien Tél/Tel: + 32 - 9 - 243 96 00 | Latvija Ipsen Pharma parstavnieciba Kalnciema iela 33-5 Riga LV 1046 Tel: +371 67622233 |
Česká republika Ipsen Pharma, s.r.o. Olbrachtova 2006/9,140 00 Praha 4 Ceská republika Tel: + 420 242 481 821 | Lietuva Ipsen Pharma SAS Lietuvos filialas
08103 Vilnius Tel. +370 700 33305 |
Danmark, Norge, Suomi/Finland, Sverige, Ísland Institut Produits Synthèse (IPSEN) AB Kista Science Tower Färögatan 33 SE- 164 51 KistaSverige/Ruotsi/Svíþjóð Tlf/Puh/Tel/Sími: +46 8 451 60 00 | Magyarország Ipsen Pharma Hungary Kft. Váci út 33. IX. em. H- 1134 Budapest Tel.: + 36-1-555-5930 |
Deutschland, Österreich Ipsen Pharma GmbH Einsteinstraße 174D-81677 München Tel: + 49 89 2620 432 89 | Nederland Ipsen Farmaceutica B.V. Taurusavenue 33b NL-2132 LS Hoofddorp Tel: + 31 (0) 23 55 41 600 |
Eesti CentralPharma Communications OÜ Selise 26–11, 13522, Tallinn Estonia Tel: +372 6015540 | Polska Ipsen Poland Sp. z o.o. Al. Jana Pawla II 29 00-867 Warszawa Tel.: + 48 (0) 22 653 68 00 |
Ελλάδα, Κύπρος,Μάλτα Ipsen Μονοπρ?σωπη EΠΕ Αγ. Δημητρ?ου 63 ?λιμος GR-17456 Αθ?να Ελλ?δα Τηλ: + 30 - 210 - 984 3324 | Portugal Ipsen Portugal - Produtos Farmacêuticos S.A. Alameda Fernão Lopes, nº 16A, 1ºB 1495-190 Algés Portugal Tel: + 351 - 21 - 412 3550 |
España Ipsen Pharma S.A. Torre Realia, Plaza de Europa 41-43 08908 L’Hospitalet de Llobregat Barcelona Tel: + 34 - 936 - 858 100 | România Ipsen Pharma România SRL Sectorul 1, Strada Grigore Alexandrescu nr. 59, Etai 1 Bucuresti, 010623 Tel: + 40 (021) 231 27 20 |
France Ipsen Pharma 65 quai Georges Gorse F-92100 Boulogne-Billancourt Tél: + 33 - 1 - 58 33 50 00 | Slovenija PharmaSwiss d.o.o. Brodišce 32 SI-1236 Trzin Tel: + 386 1 236 47 00 |
Ireland Ipsen Pharmaceuticals Ltd. Blanchardstown Industrial Park Blanchardstown IRL-Dublin 15 Tel: +353-1-809-8200 | Slovenská republika Ipsen Pharma, organizacná zložka Zámocká 3 SK-811 01 Bratislava Tel: + 420 242 481 821 |
Italia Ipsen SpA Via del Bosco Rinnovato n. 6 Milanofiori Nord Palazzo U7 20090 Assago (Mi) Tel: + 39 - 02 - 39 22 41 | United Kingdom Ipsen Ltd. 190 Bath Road Slough, Berkshire SL1 3XE Tel: + 44 (0)1753 - 62 77 00 |
???????? PharmaSwiss EOOD 16, Troyanski Prohod Street Floor 3, Office 8, Lagera 1612 Sofia ???.: +359 2 8952 110 |
Date of the last review of this prospectus:
Detailed information about this medication is available on the website of the European Medicines Agency http://www.ema.europa.eu.
Preloaded NutropinAq Pen
Instructions for use with
NutropinAq
THIS MEDICATIONSHOULDNOTBEINJECTEDUNTILTHEDOCTOR OR NURSEHASTAUGHTYOUTHEEXACTTECHNIQUE
Caution:
Before using the preloaded NutropinAq Pen, read these instructions carefully. We also recommend that you ask your doctor or nurse to teach you how to use it.
The preloaded NutropinAq Pen is designed to be used only with NutropinAq cartridges (for subcutaneous use only).
As shown in the following illustrations, the NutropinAq Pen and cartridges are available in two designs (with or without the additional yellow color). The operation of the pen and the content of the cartridges are the same for both designs. Either design of the cartridge can be used with either design of the NutropinAq Pen.
Use only the pen needles recommended by your doctor or nurse.
The dose scale next to the cartridge window should not be used as a dosing measure. It should only be used to calculate the dose remaining in the cartridge. Always consult the LCD screen instead of relying on audible clicks to prepare a NutropinAq injection. The clicks are only a sound confirmation that the black dosage dial has moved.
Always keep the pen and cartridges in a clean and safe place in the refrigerator, at a temperature between 2 and 8°C, and keep them out of the reach and sight of children. Protect them from intense light. Use a portable refrigerator to store your preloaded NutropinAq Pen when traveling. NutropinAq is formulated to withstand a maximum of one hour per day outside the refrigerator. Avoid areas with extreme temperatures. Check the expiration date of the cartridge before use.
To avoid infection, follow these safety measures:
- Wash your hands thoroughly with soap and water before using the pen.
- Clean the rubber cap of the cartridge with a cotton ball or swab impregnated with alcohol.
- Avoid touching the rubber cap of the cartridge at all times.
- If you accidentally touch the rubber cap of the cartridge, clean it again with an alcohol-impregnated swab.
- Do not use the same needle for more than one person.
- Use the needles only once.
Components of the preloaded NutropinAq Pen:
The following list includes the necessary elements to administer an injection. Gather all these components before use.
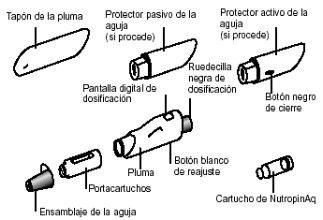
The NutropinAq cartridge and pen are supplied separately.
Part I: preparation and injection
Follow the instructions in this section if you are using the pen for the first time or are going to change an empty cartridge.
Inspect all new cartridges before use. Occasionally, after refrigeration, you may notice the presence of small, colorless particles in the NutropinAq solution, which is not uncommon in solutions containing proteins like NutropinAq and does not indicate that the product concentration has changed. Let the cartridge reach room temperature and move it gently. Do not shake it. If the solution is cloudy, unclear, or contains solid matter, do not use the cartridge. Return the cartridge to your pharmacist or primary care physician.
 1 Remove the green cap from the pen and unscrew the cartridge holder from it. If necessary, remove the empty cartridge and dispose of it properly.
1 Remove the green cap from the pen and unscrew the cartridge holder from it. If necessary, remove the empty cartridge and dispose of it properly.
- Press the white reset button.
- Turn the black dosage dial counterclockwise to set it to its initial position until it stops (see illustration). Then, turn the dosage dial clockwise until you hear a click (approximately 1/4 turn). This ensures the reset of the plunger rod, which returns to its initial position. If this step is not performed, when the dosage dial is pressed for the first time, NutropinAq may leak out or the cartridge may break.
- Insert the cartridge into the cartridge holder and then screw the cartridge holder back into the pen (be careful not to touch the rubber cap).
- Remove the paper seal from the new needle and screw it onto the cartridge holder.
- Remove the two protective caps from the needle by gently pulling them. Do not discard the larger cap, as it will be used later to remove and dispose of the needle properly.
- While holding the pen with the needle pointing upwards, gently tap the cartridge holder to move any air bubbles that may be present to the top. While continuing to hold the pen upwards, adjust the black dosage dial by inserting it until it clicks. You will see a drop of solution appear.
Be patient: if the medication does not appear after a few seconds, you may need to press the reset button again.
- If a drop of medication does not appear, press the white reset button again. Then, turn the black dosage dial clockwise (see illustration) until you hear a click (0.1 mg). If you accidentally turn it too far, turn it counterclockwise until you hear a click (0.1 mg).
- While continuing to hold the pen upwards, adjust the black dosage dial again and observe the tip of the needle to check if a drop of medication appears. Repeat steps 8 and 9 until it appears.
- Press the white reset button.
- Select the required dose by turning the black dosage dial. If you cannot select the full dose, start a new cartridge (as described in Part I) or inject a partial dose. Then, start a new cartridge (as described in Part I) to administer the remaining amount of your medication. Your doctor or nurse will advise you on the procedure for administering the last dose of the cartridge.
Clean the injection site with a cotton ball or swab impregnated with aseptic solution. Injection sites include the upper arms, abdomen, and upper thighs. Alternate injection sites to avoid discomfort. Although you may have a preference for a site, you should alternate injection sites.
 Upper arm
Upper arm
Abdomen
Thigh
- If you are using the passive protector (or not using the protector), proceed to step 13. If you are using the active protector, slide the protector onto the preloaded pen and push the two black buttons of the needle protector towards the tip.
- Place the tip of the preloaded pen on the prepared injection site and insert the needle into the skin by pushing the pen downwards until the protector is fully pressed. Your doctor or nurse will show you how to do this. At this point, you will be ready to administer the dose. Press the black dosage dial. Wait 5 seconds after pressing the button and then remove the pen from the skin. A drop of blood may appear. If desired, apply a band-aid to the injection site.
- Remove the needle protector from the pen (if used) and place the larger needle cap on a flat surface. Slide the needle into the cap to collect it and push it down, closing it completely. Unscrew the needle and dispose of it properly. Your doctor or nurse will advise you on how to dispose of the items used for the injection. Always keep the needle collection container out of the reach of children.
- Put the pen cap back on and return the pen to its case with the black dosage dial pressed inwards. The pen should always be stored in a refrigerator. Do not remove the cartridge between injections. DO NOT FREEZE.
To administer other injections with the preloaded NutropinAq Pen, put on a new needle, press the white reset button, and select your dose.
Part II: storage and maintenance
Follow these tips to ensure proper care of the preloaded NutropinAq Pen:
- Always keep the preloaded pen and NutropinAq cartridge in a refrigerator and protect them from light when not in use.
- You can remove the pen and cartridge from the refrigerator up to 45 minutes before use.
- Do not allow the preloaded pen and/or NutropinAq cartridge to freeze. Contact your doctor or nurse to replace it if the pen or cartridge is damaged.
- Avoid extreme temperatures. The solution in the cartridge is stable for 28 days after first use when stored between 2 and 8°C.
- If you need to clean the pen, do not put it in water. Use a damp cloth to remove dirt. Do not use alcohol.
- When starting a new cartridge, you may need to repeat steps 8 and 9 of Part I up to 6 times (0.6 mg) to remove any air bubbles that may be present. Small bubbles may remain without affecting the dose.
- The preloaded pen should contain the NutropinAq solution in use. Do not remove the cartridge between injections.
- The NutropinAq cartridge can be used for a maximum of 28 days.
- Do not store the preloaded NutropinAq Pen with the needle attached.
Part III: needles for the preloaded NutropinAq Pen
Your doctor or nurse will recommend a needle that is suitable for you. Always use the recommended needles.
Needles from other regions or countries may not be suitable for your preloaded NutropinAq Pen. If you travel outside the European Union, make sure to bring enough needles for the duration of your stay.
Part IV: frequently asked questions
Q: Should I change the needle every time I use the preloaded NutropinAq Pen?
A: Yes. A new needle should be used for each injection. The needle is only sterile the first time it is used.
Q: Where should I store the preloaded NutropinAq Pen?
A: The preloaded NutropinAq Pen should be stored in its case and in a refrigerator when a cartridge is inserted. When traveling, carry the preloaded pen in a portable refrigerator. DONOTFREEZE.
Q: Why do I keep the medication in the refrigerator?
A: To maintain its concentration.
Q: Can I store the preloaded NutropinAq Pen in the freezer?
A: No. Freezing would damage the pen and the medication.
Q: How long can I leave the preloaded NutropinAq Pen and the NutropinAq cartridge outside the refrigerator?
A: We recommend no more than one hour. Your doctor or nurse will advise you on the storage of the pen.
Q: What is the maximum dose that the preloaded NutropinAq Pen can administer in one injection?
A: The preloaded NutropinAq Pen can administer a minimum dose of 0.1 mg up to a maximum dose of 4.0 mg (40 clicks). If you try to dose more than 4 mg at one time, the medication may spill out of the needle and be lost, or excessive pressure may be applied to the cartridge and it may break.
Q: Can I turn the black dosage dial backwards if I have turned it too far?
A: Yes. You can turn the black dosage dial backwards until the correct number appears on the LCD screen.
Q: What should I do if the solution remaining in the cartridge is not enough for my next dose?
A: Your doctor or nurse will advise you on what to do for the last dose of the cartridge.
Q: Why do I need to turn the black dosage dial backwards every time I change the cartridge?
A: This ensures that the plunger rod returns to its initial position. If this is not done, the liquid may leak out of the needle when a new cartridge is placed in the pen.
Q: Can I use the preloaded NutropinAq Pen without the protectors?
A: Yes. The preloaded NutropinAq Pen works without the protectors, as they are optional to help you administer the injection.
Q: What should I do if I drop the preloaded NutropinAq Pen?
A: If you drop the preloaded NutropinAq Pen, check if the cartridge is damaged. You should also check the pen to determine if the black dosage dial moves up and down properly and the LCD counter works. If your cartridge or pen is damaged, ask your doctor or nurse to replace it.
Q: How long can I use the preloaded NutropinAq Pen?
A: The preloaded NutropinAq Pen is designed to last 24 months from the first time you use the pen.
Q: What do the flashing letters ‘bt’ on the LCD screen mean?
A: They mean that the batteries of the preloaded NutropinAq Pen are running out. Contact your doctor or nurse to replace them. In general, the batteries last 24 months and have 4 weeks of life from the moment the letters ‘bt’ start flashing.
Q: How do I change the preloaded NutropinAq Pen?
A: Contact your doctor or nurse if you need a replacement part or if you need to replace the entire pen.
For more information, please contact your local representative. Your local representative is the same for the NutropinAq Pen delivery device and for the medication, as detailed on the back. To contact your local representative, see section 6, on the back of this prospectus.
CE 0459
Manufacturer:IPSEN PHARMA BIOTECH S.A.S., Parc d’Activités du Plateau de Signes, CD no 402, 83870 Signes, France
Date of the last review of this prospectus:
NutropinAq is a registered trademark of Genentech, Inc.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NUTROPINAQ 10 mg/2 ml (30 IU) INJECTABLE SOLUTIONDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 0.2 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for NUTROPINAQ 10 mg/2 ml (30 IU) INJECTABLE SOLUTION
Discuss questions about NUTROPINAQ 10 mg/2 ml (30 IU) INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











