NOVOSEVEN 2 mg (100 IU) POWDER AND SOLVENT FOR INJECTION

How to use NOVOSEVEN 2 mg (100 IU) POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
NovoSeven 1mg (50KUI) powder and solvent for solution for injection
NovoSeven 2mg (100KUI) powder and solvent for solution for injection
NovoSeven 5mg (250KUI) powder and solvent for solution for injection
NovoSeven 8mg (400KUI) powder and solvent for solution for injection
eptacog alfa (activated)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is NovoSeven and what is it used for
- What you need to know before you use NovoSeven
- How to use NovoSeven
- Possible side effects
- Storage of NovoSeven
- Contents of the pack and further information
On the back: Instructions for use of NovoSeven
1. What is NovoSeven and what is it used for
NovoSeven is a blood clotting factor. It works by activating the blood clotting system in the site of bleeding when the patient's own clotting factors do not work.
NovoSeven is used to treat bleeding and prevent excessive bleeding after surgical procedures or other major treatments. Early treatment with NovoSeven reduces the amount and duration of bleeding. It works in all types of bleeding, including joint bleeding. This reduces the need for hospitalization and days off work or school.
It is used in certain groups of people:
- If you have congenital hemophilia and do not respond normally to treatment with factors VIII or IX
- If you have acquired hemophilia
- If you have Factor VII deficiency
- If you have Glanzmann's thrombasthenia (a bleeding disorder) and your disease cannot be effectively treated with platelet transfusion, or if platelets are not readily available.
NovoSeven may also be given to you by a doctor to treat heavy bleeding after childbirth, even if you do not have a bleeding disorder.
2. What you need to know before you use NovoSeven
Do not use NovoSeven
- If you are allergic to eptacog alfa (the active substance of NovoSeven) or to any of the other ingredients of this medicine (listed in section 6).
- If you are allergic to mouse, hamster or bovine proteins (such as cow's milk).
If any of these apply to you, do not use NovoSeven. Talk to your doctor.
Warnings and precautions
Before starting treatment with NovoSeven, make sure your doctor knows:
- If you have recently undergone surgery
- If you have recently suffered a crush injury
- If your arteries are narrower due to disease (atherosclerosis)
- If you have a higher risk of blood clot formation (thrombosis)
- If you have severe liver disease
- If you have severe septicemia
- If you are prone to disseminated intravascular coagulation (DIC, a condition in which blood clots form in the bloodstream) you should be closely monitored.
If any of these apply to you, tell your doctor before using this product.
Other medicines and NovoSeven
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Do not use NovoSeven at the same time as prothrombin complex concentratesor rFXIII. You should consult your doctor before using NovoSeven if you are also using factor VIII or IX.
There is limited experience of using NovoSeven with other medicines called antifibrinolytics (such as aminocaproic acid or tranexamic acid) which are also used to control bleeding. You should consult your doctor before using NovoSeven with these medicines.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using NovoSeven.
Driving and using machines
There are no studies on the effects of NovoSeven on the ability to drive and use machines. However, there is no medical reason to think it could affect your ability.
NovoSeven contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per injection, i.e. it is essentially "sodium-free".
3. How to use NovoSeven
NovoSeven powder must be reconstituted with the solvent and injected intravenously. See the detailed instructions on the back.
When to treat
Start treatment of a bleed as soon as possible, ideally within the first 2 hours.
- In case of a mild or moderate bleed, you should treat yourself as soon as possible, preferably at home.
- In case of a severe bleed you should contact your doctor. Normally, severe bleeds are treated in hospital and you can give yourself the first dose of NovoSeven on the way there.
You must not treat for more than 24 hours without consulting your doctor.
- Each time you use NovoSeven, you must inform your doctor or hospital as soon as possible.
- If the bleed is not controlled within the first 24 hours, contact your doctor immediately. You will usually need hospital attention.
Dose
The first dose should be given as soon as possible after the start of the bleed. Ask your doctor when injections should be given and how long you should continue using them.
Your doctor will decide your dose, based on your body weight, condition and type of bleed.
To get the best results, follow the prescribed dose carefully. Your doctor may change the dose.
If you have hemophilia:
The normal dose is 90 micrograms per kilogram of body weight; you can repeat the injection every 2-3 hours until the bleed is controlled.
Your doctor may recommend a single dose of 270 micrograms per kilogram of body weight. There is no clinical experience of using this single dose in people over 65 years of age.
If you have Factor VII deficiency:
The normal dose range is 15-30 micrograms per kilogram of body weight, for each injection.
If you have Glanzmann's thrombasthenia:
The usual dose is 90 micrograms (range 80 to 120 micrograms) per kilogram of body weight, for each injection.
If you inject more NovoSeven than you should
If you inject too much NovoSeven, contact your doctor immediately.
If you forget to inject a dose of NovoSeven
If you forget to inject a dose of NovoSeven or want to stop treatment, contact your doctor immediately.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Rare (may affect up to 1 in 1,000 episodes treated)
- Allergy, hypersensitivity or anaphylactic reactions. Symptoms may include skin rash, itching, redness and hives; chest noises or difficulty breathing; feeling dizzy or faint, and severe swelling of the lips or throat, or at the injection site.
- Blood clots in the arteries of the heart (which could lead to a heart attack or angina), in the brain (which could lead to a stroke) or in the intestine and kidneys. Symptoms may include severe chest pain, difficulty breathing, confusion, difficulty speaking or moving (paralysis) or abdominal pain.
Uncommon (may affect up to 1 in 100 episodes treated)
- Blood clots in the veins of the lungs, legs, liver, kidneys or at the injection site. Symptoms may include difficulty breathing, painful swelling of the leg and abdominal pain.
- Lack of efficacy or reduced response to treatment.
If you get any of these serious side effects, seek medical help immediately. Tell them that you have been using NovoSeven.
Remember to tell your doctor if you have a history of allergic reactions as you may need closer monitoring. In the vast majority of cases reported of thrombosis, patients had a predisposition to thrombotic disorders.
Other rare side effects
(may affect up to 1 in 1,000 episodes treated)
- Nausea (feeling sick)
- Headache
- Changes in some blood test and liver results.
Other uncommon side effects
(may affect up to 1 in 100 episodes treated)
- Skin rash, including rash, itching and hives
- Fever.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Agency's website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of NovoSeven
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month stated.
- Store the powder and solvent below 25°C.
- Store the powder and solvent protected from light.
- Do not freeze.
- Use NovoSeven immediately after mixing the powder with the solvent to avoid infections. If you cannot use it immediately, after mixing, you must store it in the vial with the vial adapter and with the syringe still in place, in the refrigerator between 2°C and 8°C, for a maximum of 24 hours. Do not freeze the reconstituted NovoSeven solution and keep it protected from light. Do not store the solution without consulting your doctor or nurse.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of NovoSeven
- The active substance is recombinant coagulation factor VIIa (eptacog alfa activated).
- The other components in the powder are sodium chloride, calcium chloride dihydrate, glycylglycine, polysorbate 80, mannitol, sucrose, methionine, hydrochloric acid, and sodium hydroxide. The components of the solvent are histidine, hydrochloric acid, sodium hydroxide, and water for injections.
The powder for solution for injection contains: 1 mg/vial (which corresponds to 50 KUI/vial), 2 mg/vial (which corresponds to 100 KUI/vial), 5 mg/vial (which corresponds to 250 KUI/vial), or 8 mg/vial (which corresponds to 400 KUI/vial).
After reconstitution, 1 ml of the solution contains 1 mg of eptacog alfa (activated).
1 KUI is equal to 1,000 IU (International Units).
Appearance of NovoSeven and Container Contents
The vial of powder contains white powder, and the pre-filled syringe contains a clear and colorless solution. The reconstituted solution is colorless. Do not use the reconstituted solution if particles appear or if it is discolored.
Each NovoSeven package contains:
- 1 vial with white powder for solution for injection
- 1 vial adapter
- 1 pre-filled syringe with solvent for reconstitution
- 1 plunger
Presentation: 1 mg (50 KUI), 2 mg (100 KUI), 5 mg (250 KUI), and 8 mg (400 KUI).
The current presentation is indicated on the outer packaging.
Marketing Authorization Holder and Manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd, Denmark
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for Use of NovoSeven READ THESE INSTRUCTIONS CAREFULLY BEFORE USING NOVOSEVEN NovoSeven is presented as a powder. Before injection (administration), it must be reconstituted with the solvent that accompanies it in the syringe. The solvent is a histidine solution. The reconstitution of NovoSeven must be injected into a vein (intravenous injection). The equipment in this package is designed to reconstitute and inject NovoSeven. You will also need administration equipment (catheter and butterfly needle, sterile alcohol-impregnated swabs, gauze pads, and adhesive strips). These materials are not included in the NovoSeven package. Do not use the equipment without proper training by your doctor or nurse. Always wash your hands and ensure the area around you is clean. When preparing and injecting a medication directly into a vein, it is essential to use a clean and germ-free technique (aseptic technique).An inappropriate technique can introduce germs capable of infecting the blood. Do not open the equipment until you are ready to use it. Do not use the equipment if it has been dropped or is damaged.In that case, use a new package. Do not use the equipment if it has expired.In that case, use a new package. The expiration date is printed after EXP on the carton, vial, vial adapter, and pre-filled syringe. Do not use the equipment if you suspect it is contaminated.In that case, use a new package. Do not discard any of the materials until the reconstituted solution has been injected. The equipment is for single use. | |
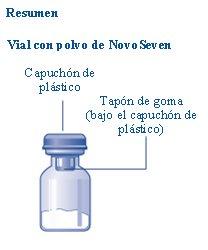
Content The package contains:
| |
| |
|
|
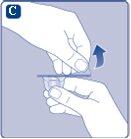
If the plastic cap is loose or missing, do not use the vial.
|
|
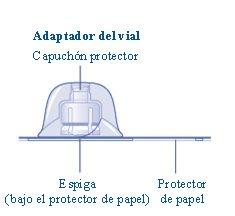
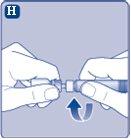
If the paper seal is not completely sealed or is broken, do not use the vial adapter. Do not remove the vial adapter from the protective cap with your fingers.If you touch the spike of the vial adapter, you can transfer germs from your fingers. |
|
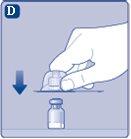
Once joined, do not remove the vial adapter from the vial. |
|
Remove the protective capfrom the vial adapter. Do not remove the vial adapter from the vialwhen removing the protective cap. |
|
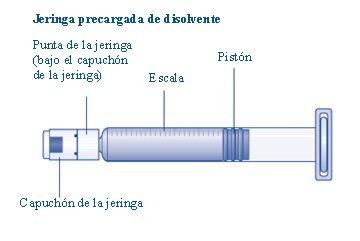
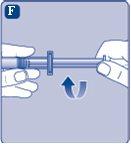
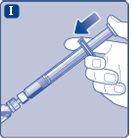
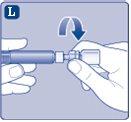
Connect the plunger immediatelyto the syringe by turning it clockwise in the piston inside the pre-filled syringe until you feel resistance. |
|
Do not touch the tip of the syringe under the syringe cap.If you touch the tip of the syringe, you can transfer germs from your fingers. If the syringe cap is loose or missing, do not use the pre-filled syringe. |
|
|
|
|
|
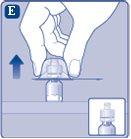
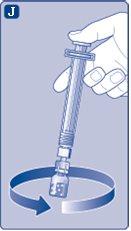
Do not shake the vial, as this can produce foam.
|
|
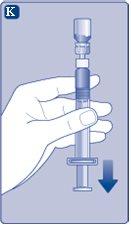
Use the reconstituted NovoSeven immediatelyto avoid infections. If you cannot use it immediately,see section 5Storage of NovoSevenon the other side of this leaflet. Do not store the reconstituted solution without consulting your doctor or nurse. (I) If your dose requires more than one vial, repeat steps A to J with additional vials, vial adapters, and pre-filled syringes until you reach the required dose. | |
|
|
|
|
Injection of NovoSeven with a pre-filled syringe through needle-free connectors for intravenous (IV) catheters Precaution:The pre-filled syringe is made of glass and is designed to be compatible with standard luer-lock connections. Some needle-free connectors with an internal spike are incompatible with the pre-filled syringe. This incompatibility can prevent administration of the medication and/or damage the needle-free connector. Follow the instructions for use of the needle-free connector. Administration through a needle-free connector may require withdrawing the reconstituted solution with a standard 10 ml sterile luer-lock plastic syringe. This should be done immediately after step J. | |
NovoSeven is now ready to be injected into a vein.
Injection through a central venous access device (CVAD) such as a central venous catheter or a subcutaneous port:
| |
Disposal
|
|
Do not disassemble the equipment before discarding it. Do not reuse the equipment. |
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NOVOSEVEN 2 mg (100 IU) POWDER AND SOLVENT FOR INJECTIONDosage form: INJECTABLE, 1 mg (45 kIU)Active substance: coagulation factor VIIaPrescription requiredDosage form: INJECTABLE, 2 mg (90 kIU)Active substance: coagulation factor VIIaPrescription requiredDosage form: INJECTABLE, 5 mg (225 kIU)Active substance: coagulation factor VIIaPrescription required
Online doctors for NOVOSEVEN 2 mg (100 IU) POWDER AND SOLVENT FOR INJECTION
Discuss questions about NOVOSEVEN 2 mg (100 IU) POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions