NORDITROPIN FLEXPRO 5 mg/1.5 ml SOLUTION FOR INJECTION IN PRE-FILLED PEN

How to use NORDITROPIN FLEXPRO 5 mg/1.5 ml SOLUTION FOR INJECTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Norditropin FlexPro 5mg/1.5ml solution for injection in pre-filled pen
somatropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Norditropin FlexPro and what is it used for
- What you need to know before you use Norditropin FlexPro
- How to use Norditropin FlexPro
- Possible side effects
- Storing Norditropin FlexPro
- Contents of the pack and other information
Instructions for use of Norditropin FlexPro
1. What is Norditropin FlexPro and what is it used for
Norditropin FlexPro contains a synthetic human growth hormone, called somatropin, which is identical to the natural growth hormone produced by the body. Children need growth hormone to help them grow, but adults also need it for their general health.
Norditropin FlexPro is used to treat growth failure in children
- If they have little or no production of growth hormone (growth hormone deficiency)
- If they have Turner syndrome (a genetic problem that can affect growth)
- If they have kidney disease
- If they are short and were born small for gestational age (SGA)
- If they have Noonan syndrome (a genetic problem that can affect growth).
Norditropin FlexPro is used as a growth hormone substitute in adults
In adults, Norditropin FlexPro is used as a growth hormone substitute when their growth hormone production has decreased since youth or has disappeared in adulthood as a result of a tumor, tumor treatment, or a disease that affects the gland that produces growth hormone. If you received treatment for growth hormone deficiency during childhood, you will be retested once you have finished growing. If growth hormone deficiency is confirmed, you should continue treatment.
2. What you need to know before you use Norditropin FlexPro
Do not use Norditropin FlexPro
- If you are allergic to somatropin, phenol, or any of the other ingredients of this medicine (listed in section 6)
- If you have had a kidney transplant
- If you have an active tumor (cancer). Tumors must be inactive and you must have completed anti-tumor therapy before starting treatment with Norditropin FlexPro
- If you have a severe acute illness, e.g., you have had open heart or abdominal surgery, have had multiple accidental injuries, or have acute respiratory failure
- If you have stopped growing (epiphyseal closure) and do not have a growth hormone deficiency.
Warnings and precautions
Talk to your doctor or pharmacist before starting Norditropin FlexPro
- If you have diabetes
- If you have had cancer or any other type of tumor
- If you have frequent headaches, vision problems, nausea, or vomiting
- If your thyroid gland is not working properly
- A sideways curvature of the spine (scoliosis) may develop in any child during rapid growth. During treatment with Norditropin FlexPro, your doctor will examine you (or your child) for signs of scoliosis.
- If you start limping during your treatment with growth hormone, tell your doctor.
- If you are over 60 years old or if, as an adult, you have been treated with somatropin for more than 5 years, as experience in these cases is limited
- If you have kidney disease, as your doctor will need to monitor your kidney function
- If you are receiving glucocorticoid replacement therapy, you should consult your doctor regularly, as your glucocorticoid dose may need to be adjusted.
- Norditropin FlexPro may cause pancreatitis, which causes severe abdominal and back pain. If you or your child develop stomach pain after administering Norditropin FlexPro, consult your doctor.
Other medicines and Norditropin FlexPro
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
In particular, tell your doctor if you are taking or have recently taken any of the following medicines. Your doctor may need to adjust the dose of Norditropin FlexPro or the other medicines:
- Glucocorticoids - your adult height may be affected if you use Norditropin FlexPro and glucocorticoids at the same time
- Ciclosporin (immunosuppressant) - you may need a dose adjustment
- Insulin - you may need a dose adjustment
- Thyroid hormones - you may need a dose adjustment
- Gonadotropin (gonad-stimulating hormone) - you may need a dose adjustment
- Anticonvulsants - you may need a dose adjustment
- Oral estrogens or other sex hormones.
Pregnancy and breastfeeding
Products containing somatropin are not recommended for fertile women who do not use contraceptive methods.
- Pregnancy. Stop treatment and tell your doctor if you become pregnant while being treated with Norditropin FlexPro
- Breastfeeding. Do not use Norditropin FlexPro during breastfeeding, as somatropin may pass into breast milk.
Driving and using machines
Norditropin FlexPro does not affect the ability to drive or use machines.
Important information about some of the ingredients of Norditropin FlexPro
This medicine contains somatropin, which may produce a positive result in doping tests.
Norditropin contains sodium
Norditropin contains less than 1 mmol of sodium (23 mg) per 1.5 ml; i.e., it is essentially "sodium-free).
3. How to use Norditropin FlexPro
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are not sure, ask your doctor or pharmacist again.
Recommended dose
The dose for children depends on their weight and body surface area. The dose in later stages of life depends on height, weight, sex, and sensitivity to growth hormone and will be adjusted until the correct dose for you is found.
- Children with growth hormone deficiency or deficiency:
The usual dose is 0.025 to 0.035 mg per kg of body weight per day or 0.7 to 1.0 mg per m2 of body surface area per day
- Girls with Turner syndrome:
The usual dose is 0.045 to 0.067 mg per kg of body weight per day or 1.3 to 2.0 mg per m2 of body surface area per day
- Children with kidney disease:
The usual dose is 0.050 mg per kg of body weight per day or 1.4 mg per m2 of body surface area per day
- Children born small for gestational age (SGA):
The usual dose is 0.035 mg per kg of body weight per day or 1.0 mg per m2 of body surface area per day until final height is reached. (In clinical trials in children with short stature born SGA, doses of 0.033 and 0.067 mg per kg of body weight per day have been used)
- Children with Noonan syndrome:
The usual dose is 0.066 mg per kg of body weight per day, however, your doctor may decide that 0.033 mg/kg/day is sufficient.
- Adults with growth hormone deficiency:
If growth hormone deficiency persists after you have finished growing, you should continue treatment. The usual starting dose is 0.2 to 0.5 mg per day. Your dose will be adjusted until you reach the correct dose. If growth hormone deficiency begins in adulthood, the usual starting dose is 0.1 to 0.3 mg per day. Your doctor will increase this dose every month until you reach the correct dose for you. The usual maximum dose is 1.0 mg per day.
When to use Norditropin FlexPro
Inject the daily dose under the skin every night before bedtime.
How to use Norditropin FlexPro
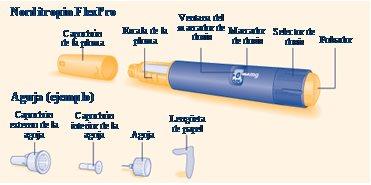
The Norditropin FlexPro growth hormone solution is available in a 1.5 ml pre-filled multidose pen.
On the back, you can find the complete instructions for using Norditropin FlexPro. The key points of the instructions are as follows:
- Check the solution before using the pen by turning it upside down one or two times. Do not use the pen if the solution is cloudy or discolored (see page 8, step A)
- Norditropin FlexPro is designed to be used with the NovoFine or NovoTwist disposable needles up to 8 mm in length
- Always use a new needle for each injection
- Vary the injection site to avoid damaging your skin
- Check the flow of growth hormone before the first injection with a new Norditropin FlexPro pen to make sure you receive the correct dose and do not inject air. Do not use the pen if a drop of growth hormone does not appear at the tip of the needle (see pages 10-11, steps E-G)
- Do not share the Norditropin FlexPro pen with anyone else.
How long will you need to continue treatment
- Children with growth failure due to Turner syndrome, kidney disease, born small for gestational age (SGA), or Noonan syndrome: your doctor will recommend that you continue treatment until you finish growing
- Children or adolescents who lack growth hormone: your doctor will recommend that you continue treatment in adulthood.
Do not stop treatment with Norditropin FlexPro unless your doctor tells you to.
If you use more Norditropin FlexPro than you should
Tell your doctorif you inject too much somatropin. Prolonged overdose may cause abnormal growth and deformation of facial features.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested
If you forget to use Norditropin FlexPro
Use the next dose as usual, at the usual time. Do not use a double doseto make up for forgotten doses.
If you stop treatment with Norditropin FlexPro
Do not stop treatment with Norditropin FlexPro unless your doctor tells you to.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects seen in children and adults(frequency not known)
- Skin rash, difficulty breathing, swollen face, lips, or eyelids, collapse. Any of these symptoms may be a sign of an allergic reaction
- Headache, vision problems, nausea, and vomiting. These may be symptoms of increased pressure in the brain
- Decreased levels of thyroxine in the blood
- Hyperglycemia (high blood sugar levels).
If you experience any of these side effects, consult your doctor as soon as possible. Stop using Norditropin FlexPro until your doctor tells you that you can continue treatment.
Antibodies to somatropin have been rarely reported during treatment with Norditropin.
Increased liver enzyme levels have been rarely reported.
Cases of leukemia and recurrence of brain tumors have been reported in patients treated with somatropin (the active ingredient in Norditropin FlexPro), although there is no evidence that somatropin is responsible for this.
If you think you may be suffering from any of these diseases, talk to your doctor.
Other side effects in children
Uncommon(may affect up to 1 in 100 children)
- Headache
- Redness, itching, and pain at the injection site
- Increased size of the breasts (gynecomastia).
Rare(may affect up to 1 in 1,000 children)
- Skin rash
- Muscle and joint pain
- Swollen hands and feet due to fluid retention.
In rare cases, children treated with Norditropin FlexPro have experienced hip or knee pain or have started limping. These symptoms may be due to a disease that affects the upper end of the femur (Legg-Calve-Perthes disease) or because the end of the bone has slipped out of the cartilage (slipped capital femoral epiphysis) and may not be due to Norditropin FlexPro.
In clinical trials, some cases of increased growth of hands and feetin relation to height have been observed in children with Turner syndrome.
In a clinical trial in children with Turner syndrome, it has been observed that high doses of Norditropin may possibly increase the risk of ear infections.
If you think any of the side effects you are experiencing are serious, or if you notice any side effects not mentioned in this leaflet, tell your doctor or pharmacist, as your dose may need to be reduced.
Other side effects in adults
Very common(may affect more than 1 in 10 adults)
- Swollen hands and feet due to fluid retention.
Common(may affect up to 1 in 10 adults)
- Headache
- Feeling of pins and needles and numbness or pain, mainly in the fingers
- Pain and stiffness in the joints; muscle pain.
Uncommon(may affect up to 1 in 100 adults)
- Type 2 diabetes
- Carpal tunnel syndrome; tingling and pain in the fingers and hands
- Itching (may be intense) and pain at the injection site
- Muscle stiffness
- Increased size of the breasts (gynecomastia).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Norditropin FlexPro
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month stated.
Storeunused Norditropin FlexPro pens in a refrigerator(between 2°C and 8°C) in the outer packaging to protect them from light. Do not freeze or expose to heat. Do not store near the wall or the cooling element of the refrigerator.
While usingNorditropin FlexPro 5 mg/1.5 ml you can:
- Store it in a refrigerator (between 2°C and 8°C) for a maximum of 4 weeks, or
- Store it at room temperature (below 25°C) for a maximum of 3 weeks.
Do not use Norditropin FlexPro pens if they have been frozen or exposed to excessive temperatures.
Do not use Norditropin FlexPro pens if the growth hormone solution is cloudy or discolored.
Always keep Norditropin FlexPro without the needle attached.
Always keep the pen cap completely closed on the Norditropin FlexPro pen when not in use.
Always use a new needle for each injection.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Norditropin FlexPro
- The active ingredient is somatropin
- The other components are mannitol, histidine, poloxamer 188, phenol, water for injectable preparations, hydrochloric acid, and sodium hydroxide.
Appearance of the Product and Container Contents
Norditropin FlexPro is a clear and colorless injectable solution in a 1.5 ml pre-filled multidose disposable pen.
1 ml of solution contains 3.3 mg of somatropin.
1 mg of somatropin corresponds to 3 IU of somatropin.
Norditropin FlexPro is available in three concentrations:
5 mg/1.5 ml, 10 mg/1.5 ml, and 15 mg/1.5 ml (equivalent to 3.3 mg/ml, 6.7 mg/ml, and 10 mg/ml, respectively) in a package size of 1 or 5 pre-filled pens. Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Novo Nordisk A/S
Novo Allé, 1
DK-2880 Bagsværd, Denmark
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder.
Local Representative:
Novo Nordisk Pharma, S.A.
Vía de los Poblados, 3, Edificio 6
28033, Madrid
Spain
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany, Austria, Belgium, Cyprus, Denmark, Slovenia, Finland, Greece, Ireland, Lithuania, Luxembourg, Malta, Netherlands, Portugal, United Kingdom (Northern Ireland), Romania: Norditropin FlexPro 5 mg/1.5 ml
Sweden: Somatropin Novo Nordisk 5 mg/1.5 ml
France: Norditropine FlexPro 5 mg/1.5 ml
Date of Last Revision of this Leaflet:March 2025
Other Sources of Information
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
Instructions for Use of Norditropin FlexPro Read these instructions carefully before using the Norditropin FlexPro pen. Start by checking the name, concentration, and color of the labelon the Norditropin FlexPro pen to ensure it contains the growth hormone concentration you need. Continue reading to learn about: Preparing the Norditropin FlexPro Pen Checking the Growth Hormone Flow with Each New Pen Selecting the Dose Injecting the Dose Caring for the Norditropin FlexPro Pen Important Information | |
| |
The Norditropin FlexPro pen is a pre-filled growth hormone pen. Norditropin FlexPro contains 5 mg of growth hormone solution and delivers doses from 0.025 mg to 2.0 mg, in increments of 0.025 mg. Norditropin FlexPro is designed to be used with the NovoFine or NovoTwist disposable needles of up to 8 mm in length. | |
Preparing the Norditropin FlexPro Pen Check the name, concentration, and color of the labelon the Norditropin FlexPro pen to ensure it contains the growth hormone concentration you need. | |
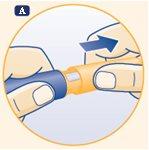
A. Remove the pen cap. Check that the growth hormone solution in the pen is clear and colorless by turning it upside down one or two times. If the solution appears unclear or cloudy, do not use the pen. |
|
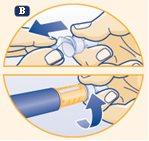
B. Take a new disposable needle. Remove the paper tab from the needle and screw the straight needle onto the pen. Make sure the needle is tightened. |
|
Always use a new needle for each injection. This reduces the risk of contamination, infection, loss of growth hormone, needle blockage, and inaccurate dosing. Never use a bent or damaged needle. | |
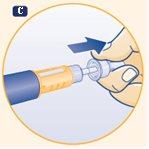
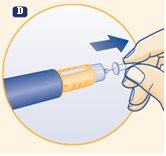
C. Remove the outer needle cap and set it aside. After injection, you will need it to properly remove the needle from the pen. |
|
D. Remove the inner needle cap by pulling on the central tip and discard it. If you try to put it back on, you may accidentally prick yourself with the needle. A drop of growth hormone may appear on the tip of the needle. This is normal. |
|
Checking the Growth Hormone Flow with Each New Pen Make sure you receive the full dose by checking the growth hormone flow before selecting and injecting the first dose with each new pen. | |
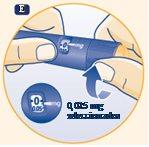
E. Turn the dose selector to select the minimumdose, 0.025 mg. |
|
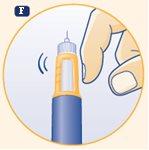
F. Hold the pen with the needle pointing upwards. Tap the top of the pen several times to make any air bubbles rise to the top. |
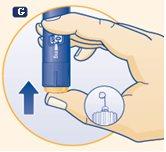
|
G. Press the push button until the 0 is aligned with the dose marker and a drop of growth hormone appears on the tip of the needle. If no drop appears, repeat steps E to G a maximum of 6 times. If still no drop appears, change the needle and repeat steps E to G once more. Do not use the pen if a drop of growth hormone still does not appear. |
|
Always make sure a drop appears on the tip of the needle before injecting the first dose with each new pen. | |
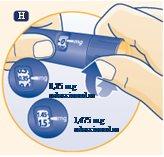
Selecting the Dose Use the dose selector on the Norditropin FlexPro pen to select up to 2.0 mg per dose. | |
H. Select or adjust the required dose by turning the dose selector forward or backward until the correct number of mg is aligned with the dose marker. When the pen contains less than 2.0 mg, the dose selector stops at the number of mg remaining. |
|
| |
The pen scale can be used to see approximately how much growth hormone is left in the pen. You can use the dose selector to see how much growth hormone is left – if the pen contains less than 2.0 mg: Turn the dose selector until it stops. The number that is aligned with the dose selector shows how many mg are left. If you need more growth hormone than is left in the pen, you can use a new pen or divide the dose between the current pen and the new one. | |
Never use the pen clicks to count the number of mg you select. Only the dose marker and the dose marker window will indicate the exact number of mg. Never use the remaining growth hormone scale to measure how much growth hormone is injected. Only the dose marker and the dose marker window will indicate the exact number of mg. | |
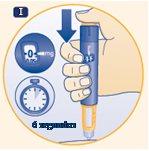
Injecting the Dose Make sure you receive the full dose by using the correct injection technique. | |
I. Insert the needle under the skin as your doctor or nurse has taught you. Press the push button to inject until the 0 is aligned with the dose marker in the dose marker window. You may hear or feel a click when doing this. Leave the needle under the skin for at least 6secondsto ensure you receive the full dose. You can release the push button while waiting. |
|
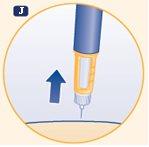
J. Remove the needle from the skin. After this, a drop of growth hormone may appear on the tip of the needle. This is normal and does not affect the dose you just received. |
|
Never use the pen clicks to count the number of mg you inject. Only the dose marker and the dose marker window will indicate the exact number of mg. Never touch the dose marker window during injection as you may block the injection. | |
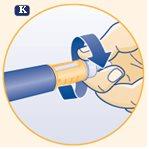
K. Carefully replace the outer needle cap without touching the needle. Unscrew the needle and dispose of it carefully according to the instructions given by your doctor or nurse. Replace the pen cap after each use. When the pen is empty, dispose of it without the needle as recommended by your doctor or nurse and according to local regulations. |
|
Never put the inner needle cap back on the needle once you have removed it. You may accidentally prick yourself with the needle. Always keep the pen without the needle attached. This reduces the risk of contamination, infection, loss of growth hormone, needle blockage, and inaccurate dosing. | |
Caring for the Norditropin FlexPro Pen Handle the Norditropin FlexPro pen with care:
| |
Important Information
| |
Important Information Pay special attention to these notes as they are important for the safe use of the pen.
| |
Norditropin FlexPro 5 mg/1.5 ml somatropin © 2025 Novo Nordisk A/S |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NORDITROPIN FLEXPRO 5 mg/1.5 ml SOLUTION FOR INJECTION IN PRE-FILLED PENDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 0.2 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for NORDITROPIN FLEXPRO 5 mg/1.5 ml SOLUTION FOR INJECTION IN PRE-FILLED PEN
Discuss questions about NORDITROPIN FLEXPRO 5 mg/1.5 ml SOLUTION FOR INJECTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions