How to use NORDIMET 10 mg Injectable Solution in Pre-filled Pen
Introduction
Package Leaflet: Information for the User
Nordimet 7.5 mg solution for injection in pre-filled pen
Nordimet 10 mg solution for injection in pre-filled pen
Nordimet 12.5 mg solution for injection in pre-filled pen
Nordimet 15 mg solution for injection in pre-filled pen
Nordimet 17.5 mg solution for injection in pre-filled pen
Nordimet 20 mg solution for injection in pre-filled pen
Nordimet 22.5 mg solution for injection in pre-filled pen
Nordimet 25 mg solution for injection in pre-filled pen
methotrexate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Nordimet and what is it used for
- What you need to know before you use Nordimet
- How to use Nordimet
- Possible side effects
- Storage of Nordimet
- Contents of the pack and other information
1. What is Nordimet and what is it used for
Nordimet contains the active substance methotrexate, which:
- reduces inflammation or swelling, and
- reduces the activity of the immune system (the body's defense mechanism). An overactive immune system has been linked to inflammatory diseases.
Nordimet is a medicine used to treat several inflammatory diseases:
- Active rheumatoid arthritis in adults. Active rheumatoid arthritis is an inflammatory disease that affects the joints.
- Severe active juvenile idiopathic arthritis in five or more joints (hence this disorder is also called polyarthritis), in patients who have had an inadequate response to non-steroidal anti-inflammatory drugs (NSAIDs).
- Moderate to severe plaque psoriasis in adults who are candidates for systemic therapy, as well as severe psoriasis that also affects the joints (psoriatic arthritis) in adult patients.
- Induction of remission in adults with moderate steroid-dependent Crohn's disease, in combination with corticosteroids.
- Maintenance of remission of Crohn's disease in adults who have responded to methotrexate, as monotherapy.
2. What you need to know before you use Nordimet
Do not use Nordimet if:
- you are allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6).
- you have severe kidney disease (your doctor can tell you if you have severe kidney disease).
- you have severe liver disease (your doctor can tell you if you have severe liver disease).
- you have blood disorders.
- you have a high consumption of alcohol.
- you have a weakened immune system.
- you have a severe or existing infection, such as tuberculosis or HIV.
- you have gastrointestinal ulcers.
- you are pregnant or breastfeeding (see the section "Pregnancy, breastfeeding and fertility").
- you are receiving live vaccine at the same time.
Warnings and precautions
Acute pulmonary hemorrhage has been reported with methotrexate in patients with underlying rheumatologic disease. If you observe blood when coughing or spitting, you should contact your doctor immediately.
An increase in lymph node size (lymphoma) may occur, in which case treatment should be discontinued.
Diarrhea can be a toxic effect of Nordimet and requires discontinuation of treatment.
If you have diarrhea, talk to your doctor.
Certain brain disorders (encephalopathy/leukoencephalopathy) have been reported in cancer patients receiving methotrexate. These side effects cannot be ruled out when using methotrexate to treat other diseases.
If you, your partner, or your caregiver notice the onset or worsening of neurological symptoms, such as general muscle weakness, vision changes, changes in thinking, memory, and orientation that cause confusion and changes in personality, contact your doctor immediately because these may be symptoms of a rare and serious brain infection called progressive multifocal leukoencephalopathy (PML).
Methotrexate can make your skin more sensitive to sunlight. Avoid intense sunlight and do not use sunbeds or UV lamps without medical advice. To protect your skin from intense sunlight, wear suitable clothing or use a sunscreen with a high protection factor.
Important warning about the administration of Nordimet
Methotrexate should only be used for the treatment of rheumatic, skin, and Crohn's disease once a week. Incorrect administration of methotrexate can cause serious side effects that can be fatal. Read section 3 of this leaflet carefully.
Talk to your doctor before starting to use Nordimet if:
- you have diabetes mellitus and are being treated with insulin;
- you have prolonged inactive infections (e.g., tuberculosis, hepatitis B or C, herpes zoster);
- you have/have had any liver or kidney disease;
- you have problems with lung function;
- you are severely overweight;
- you have an abnormal accumulation of fluid in the abdomen or in the space between the lungs and the chest wall (ascites, pleural effusions);
- you are dehydrated or have a disorder that causes dehydration (e.g., dehydration due to vomiting, diarrhea, or inflammation of the mouth and lips).
If you have experienced skin problems after radiation therapy (radiation-induced dermatitis) or skin burns, these changes may reappear when taking Nordimet.
Children, adolescents, and elderly patients
Dosing instructions depend on the patient's body weight.
Use in children under 3 years of age is not recommended due to insufficient experience with the use of this medicine in this age group.
Children, adolescents, and elderly patients treated with Nordimet should be closely monitored by their doctor to identify potential side effects as soon as possible.
The dose for elderly patients should be reduced due to age-related decreases in liver and kidney function.
Special precautions for treatment with Nordimet
Methotrexate temporarily affects semen and egg production. Methotrexate can cause abortions and severe birth defects. If you are a woman, you should avoid becoming pregnant while taking methotrexate and for at least 6 months after stopping treatment with methotrexate. If you are a man, you should avoid fathering a child while taking methotrexate and for at least 3 months after stopping treatment.
See also the section "Pregnancy, breastfeeding and fertility".
Skin changes caused by psoriasis may worsen during treatment with Nordimet if exposed to ultraviolet radiation.
Recommended follow-up examinations and precautions
Even when methotrexate is used in low doses, serious side effects can occur. To detect them in time, your doctor will perform follow-up examinations and laboratory tests.
Before starting treatment:
Before starting treatment, you will have a blood test to determine if you have enough blood cells. You will also have a blood test to check liver function and to detect hepatitis. Additionally, serum albumin levels (a blood protein), hepatitis status, and kidney function will be checked. Your doctor may also decide to perform other liver tests, which may involve imaging of the liver or taking a small tissue sample from the liver for a more thorough examination. Your doctor may also check if you have tuberculosis and perform a chest X-ray or lung function test.
During treatment:
Your doctor may perform the following examinations:
- Examination of the oral cavity and pharynx to detect changes in the mucosa, such as inflammation or ulcers.
- Blood tests/complete blood count with blood cell count and measurement of serum methotrexate levels.
- Blood tests to monitor liver function.
- Imaging tests to monitor liver status.
- Taking a small tissue sample from the liver for a more thorough examination.
- Blood tests to monitor kidney function.
- Monitoring of the respiratory tract and, if necessary, lung function test.
It is very important that you attend these scheduled examinations.
If the results of some of these tests are abnormal, your doctor will adjust the treatment accordingly.
Other medicines and Nordimet
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
It is especially important that you tell your doctor if you are taking:
- other treatments for rheumatoid arthritis or psoriasis, such as leflunomide, sulfasalazine (a medicine that, in addition to being used for arthritis and psoriasis, is also used to treat ulcerative colitis), acetylsalicylic acid, phenylbutazone, or aminopyrine;
- cyclosporin (to suppress the immune system);
- azathioprine (used to prevent organ rejection after a transplant);
- retinoids (used to treat psoriasis and other skin disorders);
- anticonvulsants (used to prevent seizures), such as phenytoin, valproate, or carbamazepine;
- cancer treatments;
- barbiturates (sleeping injection);
- tranquilizers;
- oral contraceptives;
- probenecid (used to treat gout);
- antibiotics (e.g., penicillin, glycopeptides, trimethoprim-sulfamethoxazole, sulfonamides, ciprofloxacin, cefalotin, tetracyclines, chloramphenicol);
- pyrimethamine (used to prevent and treat malaria);
- vitamin preparations with folic acid;
- proton pump inhibitors (medicines that reduce stomach acid production and are used to treat heartburn or severe ulcers), such as omeprazol or pantoprazol;
- theophylline (used to treat asthma);
- cholestyramine (used to treat high cholesterol, pruritus, or diarrhea);
- NSAIDs, non-steroidal anti-inflammatory drugs (used to treat pain or inflammation);
- p-aminobenzoic acid (used to treat skin disorders);
- any live vaccine (should be avoided), such as vaccines for measles, mumps, flu, and yellow fever;
- metamizole (synonyms: novaminsulfon and dipyrone) (medicine for severe pain and/or fever);
- nitrous oxide (a gas used in general anesthesia)
Nordimet with food, drinks, and alcohol
During treatment with Nordimet, you should avoid consuming alcohol and excessive consumption of coffee, caffeinated soft drinks, and black tea, as they may increase side effects or interfere with the efficacy of Nordimet. Also, make sure to drink plenty of fluids during treatment with Nordimet, as dehydration (reduction of body water) can increase the toxicity of Nordimet.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Pregnancy
Do not use Nordimet during pregnancy or if you are trying to become pregnant. Methotrexate can cause birth defects, harm the fetus, or cause miscarriages. It is associated with malformations of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that methotrexate is not administered to pregnant women or women who plan to become pregnant. In women of childbearing age, any possibility of pregnancy should be excluded with appropriate measures, such as a pregnancy test, before starting treatment. You should avoid becoming pregnant while taking methotrexate and for at least 6 months after stopping treatment by using reliable contraceptive methods during this time (see also the section "Warnings and precautions").
If you become pregnant during treatment or think you may be pregnant, talk to your doctor as soon as possible. They should inform you about the risk of harmful effects on the fetus during treatment.
If you want to become pregnant, you should talk to your doctor, who may refer you to a specialist before the planned start of treatment.
Breastfeeding
Do not breastfeed during treatment, as methotrexate passes into breast milk. If your doctor considers that treatment with methotrexate is absolutely necessary during breastfeeding, you should stop breastfeeding.
Male fertility
Available evidence does not indicate an increased risk of malformations or miscarriages if the father takes less than 30 mg/week of methotrexate. However, it is not possible to completely rule out some risk. Methotrexate can be genotoxic. This means that the medicine can cause genetic mutations. Methotrexate can affect sperm production and cause birth defects. For this reason, you should avoid fathering a child or donating sperm while taking methotrexate and for at least 3 months after stopping treatment.
Driving and using machines
Side effects that affect the central nervous system, such as fatigue and dizziness, can occur during treatment with Nordimet. In some cases, the ability to drive vehicles and/or operate machines may be reduced. If you feel fatigued or dizzy, you should not drive or use machines.
Nordimet contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per dose, which is essentially "sodium-free".
3. How to use Nordimet
Important dosage warning for Nordimet
Use Nordimet only once a weekfor the treatment of rheumatoid arthritis, active juvenile idiopathic arthritis, psoriasis, psoriatic arthritis, and Crohn's disease, which requires weekly administration. Overuse of Nordimet can be fatal. Read section 3 of this leaflet carefully. If you have any doubts, consult your doctor or pharmacist before taking this medication.
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Nordimet is administered only once a week. You and your doctor can agree on which day of the week you will receive the injection each week.
Incorrect administration of Nordimet can produce serious adverse effects that can be fatal.
The recommended dose is:
Dose in patients with rheumatoid arthritis
The initial recommended dose is 7.5 mg of methotrexate once a week.
Your doctor may increase the dose if the dose used is not effective, but is well tolerated. The average weekly dose is 15-20 mg. Generally, a weekly dose of more than 25 mg should not be exceeded. When Nordimet starts to work, your doctor may gradually reduce the dose to the lowest effective maintenance dose possible.
Improvement of symptoms is usually expected after 4-8 weeks of treatment. Symptoms may return if treatment with Nordimet is interrupted.
Use in adults with moderate to severe plaque psoriasis or severe psoriatic arthritis
Your doctor will administer a single test dose of 5-10 mg to assess potential adverse effects.
If the test dose is well tolerated, treatment will continue after one week with an approximate dose of 7.5 mg.
A response to treatment can usually be expected after approximately 2-6 weeks. Depending on the treatment effects and blood and urine test results, treatment will continue or be discontinued.
Dose in adult patients with Crohn's disease
Your doctor will start treatment with a weekly dose of 25 mg. A response to treatment can usually be expected after 8-12 weeks. Depending on the treatment effects, your doctor may decide to reduce the dose to 15 mg per week at a later time.
Use in children and adolescents under 16 years of age with polyarticular forms of juvenile idiopathic arthritis
The doctor will calculate the necessary dose based on the child's body surface area (m2), and the dose is expressed in mg/m2.
Use in children under 3 years of age is not recommended, as there is not enough experience in this age group.
Method and duration of administration
Nordimet is administered via a subcutaneous injection. It should be injected once a week, and it is recommended to always inject Nordimet on the same day of the week.
At the beginning of your treatment, a medical professional may inject Nordimet for you. However, your doctor may decide that you can learn to inject Nordimet yourself. You will receive adequate training to do this. Under no circumstances should you attempt to self-inject unless you have been taught how to do so.
The duration of treatment will be determined by the treating physician. Treatment of rheumatoid arthritis, juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, and Crohn's disease with Nordimet is long-term treatment.
Information on how to self-inject Nordimet
If you have difficulty handling the pen, consult your doctor or pharmacist. Do not attempt to self-inject if you have not been taught how to do so. If you are unsure, consult your doctor or nurse immediately.
Before self-injecting Nordimet
- Check the expiration date of the medication. Do not use it if it has expired.
- Check that the pen is not damaged and that the medication is a clear, yellowish solution. Otherwise, use another pen.
- Examine the site of the last injection to check for redness, skin color changes, swelling, or suppuration, or if it still hurts. If so, talk to your doctor or nurse.
- Decide where you will inject the medication. Change the injection site each time.
Instructions on how to self-inject Nordimet
- Wash your hands thoroughly with water and soap.
- Sit or lie down in a comfortable and relaxed position. Make sure you can see the area of skin where you will inject.
- The pen is pre-loaded and ready to use. Visually inspect the pen. You should be able to see a yellow liquid through the viewing window. You may see a small air bubble; this will not affect the injection and will not harm you.
A drop may appear at the tip of the needle. This is normal.
- Choose an injection site and clean it with the alcohol swab provided. It takes between 30 and 60 seconds to take effect. Suitable areas for injection are the skin on the front of the abdominal wall and the skin on the front of the thigh.
- While holding the body of the pen, remove the green protective cap by gently pulling it straight out of the unit. Do not twist or bend it.
After removing the cap, hold the pen in your hand. Avoid letting the pen come into contact with any other object. This ensures that the pen is not accidentally activated and that the needle remains clean.
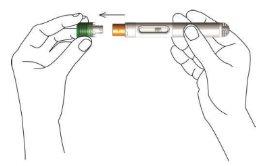
- Form a fold in the skin by gently pinching the skin at the injection site with your index and thumb fingers. Make sure to hold the skin fold during the entire injection.
- Move the pen towards the skin fold (injection site) with the needle protector pointing directly at the injection site. Place the yellow needle protector against the injection site, so that the entire edge of the needle protector is in contact with the skin.
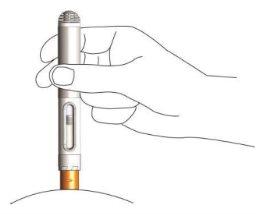
- Apply downward pressure with the pen on the skin until you hear and feel a click.
This will activate the pen, and the solution will be automatically injected into the skin.
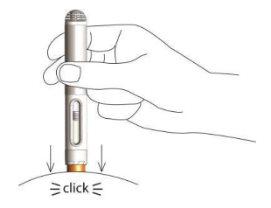
- The injection lasts a maximum of 10 seconds. You will notice and hear a second click once the injection is complete.
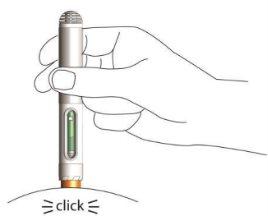
- Wait 2-3 seconds before removing the pen from the skin. The pen's safety protector will lock to prevent needlestick injuries. You can now release the skin fold.
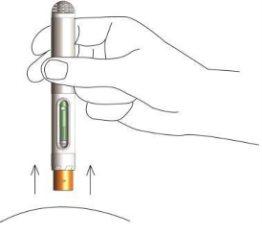
- Visually inspect the pen through the viewing window. You should be able to see a green plastic. This means that all the liquid has been injected. Dispose of the used pen in the provided sharps container. Close the container lid tightly and keep it out of the reach of children. In case of accidental contact of methotrexate with the skin or soft tissues, rinse the area with plenty of water.
If you use more Nordimet than you should
Follow your doctor's dosage recommendations. Do not change the dose without consulting your doctor.
If you suspect that you have used more Nordimet than you should, inform your doctor or immediately contact the nearest hospital. Go to the doctor's office or hospital with the medication packaging and this leaflet.
An overdose of methotrexate can cause serious toxic reactions. Overdose symptoms may include rapid bruising or bleeding, unusual weakness, mouth sores, nausea, vomiting, black or bloody stools, coughing up blood or vomit with a coffee grounds appearance, and decreased urination (emptying of the bladder). See also section 4.
If you forget to use Nordimet
Do not use a double dose to make up for forgotten doses, but continue using the prescribed dose as usual. Consult your doctor if you have doubts.
If you interrupt treatment with Nordimet
Do not stop or suspend treatment with Nordimet before discussing it with your doctor. If you suspect that you are experiencing adverse effects, consult your doctor immediately.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor if you experience sudden dizziness, difficulty breathing, swelling of the eyelids, face, or lips, skin rash, or itching (which affects your whole body).
Serious side effects
If you develop any of the following side effects, contact your doctor immediately:
- Lung inflammation (symptoms can be general illness, dry and irritating cough, difficulty breathing, shortness of breath at rest, chest pain, or fever).
- Blood when coughing or spitting.
- Severe skin peeling or blisters.
- Bleeding (including blood in vomit) or unusual bruising.
- Severe diarrhea.
- Mouth ulcers.
- Black or tarry stools.
- Blood in the urine or stools.
- Small red spots on the skin.
- Fever.
- Yellowing of the skin (jaundice).
- Pain and difficulty urinating.
- Thirst and/or frequent urination.
- Seizures (convulsions).
- Loss of consciousness.
- Vision loss or blurred vision.
The following side effects have been reported:
Very common(may affect more than 1 in 10 people)
Loss of appetite, nausea (feeling sick), stomach pain, inflammation of the mouth lining, abnormal digestion, and increased liver enzymes.
Common(may affect up to 1 in 10 people)
Reduced formation of red blood cells (hematites) with decreased white blood cells (leukocytes) and/or hematites and/or platelets (thrombocytes) (leukopenia, anemia, thrombocytopenia), headache, fatigue, drowsiness, lung inflammation (pneumonia) with dry and non-productive cough, difficulty breathing, and fever, mouth ulcers, diarrhea, skin rash, skin redness, and itching.
Uncommon(may affect up to 1 in 100 people)
Decreased number of hematites and thrombocytes, inflammation of the throat, dizziness, confusion, depression, inflammation of blood vessels, ulcers and bleeding in the digestive tract, inflammation of the intestines, vomiting, inflammation of the pancreas, liver disorders, diabetes, decreased blood proteins, herpetic skin rash, rashes, reactions similar to sunburn due to increased skin sensitivity to sunlight, hair loss, increased rheumatoid nodules, skin ulcers, shingles, joint or muscle pain, osteoporosis (reduced bone mass), inflammation and ulcers of the bladder (possibly with blood in the urine), reduced kidney function, painful urination, inflammation and ulcers in the vagina.
Rare(may affect up to 1 in 1,000 people)
Infection (including reactivation of an inactive chronic infection), septicemia, red eyes, allergic reactions, anaphylactic shock, decreased number of antibodies in the blood, inflammation of the pericardium, fluid accumulation in the pericardium, obstruction of cardiac filling due to fluid in the pericardium, visual disturbances, mood swings, low blood pressure, blood clots, scarring of lung tissue (pulmonary fibrosis), Pneumocystis jirovecii pneumonia, breathing stoppage, asthma, fluid accumulation in the pleura, gum inflammation, acute hepatitis (liver inflammation), skin darkening, acne, red or purple spots due to bleeding in blood vessels, allergic inflammation of blood vessels, bone fractures, kidney failure, decreased or absent urination, electrolyte disorders, fever, slow wound healing.
Very rare(may affect up to 1 in 10,000 people)
Reduced number of certain white blood cells (agranulocytosis), severe bone marrow failure, liver failure, inflammation of the glands, insomnia, pain, muscle weakness, numbness or tingling, changes in taste (metallic taste), seizures, inflammation of the brain lining with paralysis or vomiting, vision changes, retinal damage, vomiting blood, toxic megacolon (enlargement of the large intestine associated with severe pain), defective sperm formation (oligospermia), Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell syndrome), increased nail pigmentation, loss of libido, erection problems, infection around the nails, serious digestive tract complications, boils, visible increase in small blood vessels in the skin, menstrual disorders, vaginal discharge, infertility, breast enlargement in men (gynecomastia), and lymphoproliferative disorders (excessive growth of white blood cells).
Frequency not known(cannot be estimated from the available data)
Increased number of certain white blood cells (eosinophilia), certain brain disorders (encephalopathy/leukoencephalopathy), nosebleeds, pulmonary bleeding, jawbone damage (secondary to excessive white blood cell growth), protein in the urine, feeling of weakness, tissue destruction at the injection site, skin redness and peeling, inflammation.
Only mild local skin reactions (such as burning sensations, erythema, swelling, discoloration, intense itching, and pain) were observed with Nordimet, which decreased during treatment.
Nordimet may cause a decrease in the number of white blood cells and may decrease your resistance to infections. If you suffer from an infection with symptoms such as fever and severe deterioration of your general health, or fever with symptoms of local infection such as sore throat/pharynx/mouth, or urinary problems, you should consult your doctor immediately. They will perform a blood test to check for a possible decrease in white blood cells (agranulocytosis). It is important that you inform your doctor if you are taking Nordimet.
Methotrexate is known to cause bone disorders such as joint and muscle pain and osteoporosis. The frequency of these risks in children is unknown.
Nordimet may cause serious side effects (sometimes potentially fatal). Your doctor will perform tests to check for abnormalities that develop in the blood (e.g., low white blood cell count, low platelet count, lymphoma) and changes in the kidneys and liver.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nordimet
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the label of the pre-loaded pen and on the carton after EXP. The expiration date is the last day of the month indicated.
Store at a temperature below 25 °C.
Keep the pen in the outer packaging to protect it from light.
Do not freeze.
Do not use this medicine if you notice that the solution is not clear or contains particles.
Nordimet is for single use. Any used pen must be discarded. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Container Content and Additional Information
Nordimet Composition
The active ingredient is methotrexate. 1.0 ml of solution contains 25 mg of methotrexate.
The other components are sodium chloride, sodium hydroxide, and water for injectable preparations.
The following pre-filled pens are available:
Pre-filled pen of 0.3 ml with 7.5 mg of methotrexate
Pre-filled pen of 0.4 ml with 10 mg of methotrexate
Pre-filled pen of 0.5 ml with 12.5 mg of methotrexate
Pre-filled pen of 0.6 ml with 15 mg of methotrexate
Pre-filled pen of 0.7 ml with 17.5 mg of methotrexate
Pre-filled pen of 0.8 ml with 20 mg of methotrexate
Pre-filled pen of 0.9 ml with 22.5 mg of methotrexate
Pre-filled pen of 1.0 ml with 25 mg of methotrexate
Appearance of Nordimet and Container Content
The pre-filled pens with Nordimet contain a clear and yellowish injectable solution.
Nordimet is available in packs containing 1 or 4 pre-filled pens and 1 or 4 alcohol-impregnated swabs, and in multiple packs containing 4 or 6 boxes, each containing 1 pre-filled pen and an alcohol-impregnated swab. Nordimet is also available in multiple packs containing 3 boxes (with 4 pre-filled pens and cotton swabs).
Only some pack sizes may be marketed.
Marketing Authorization Holder
Nordic Group B.V.
Siriusdreef 41
2132 WT Hoofddorp
Netherlands
Manufacturer
CENEXI - Laboratoires Thissen
Rue de la Papyrée 2-6
B-1420 Braine-l’Alleud
Belgium
Sever Pharma Solutions AB
Agneslundsvagen 27
P.O. Box 590
SE-201 25 Malmo
Sweden
FUJIFILM Diosynth Biotechnologies Denmark ApS
Biotek Allé 1
3400 Hillerød
Denmark
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.

How much does NORDIMET 10 mg Injectable Solution in Pre-filled Pen cost in Spain ( 2026)?
The average price of NORDIMET 10 mg Injectable Solution in Pre-filled Pen in January, 2026 is around 53.56 EUR. Prices may vary depending on the region, pharmacy, and whether a prescription is required. Always check with a local pharmacy or online source for the most accurate information.
- Country of registration
- Average pharmacy price53.56 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NORDIMET 10 mg Injectable Solution in Pre-filled PenDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Alternatives to NORDIMET 10 mg Injectable Solution in Pre-filled Pen in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to NORDIMET 10 mg Injectable Solution in Pre-filled Pen in Польша
Alternative to NORDIMET 10 mg Injectable Solution in Pre-filled Pen in Украина
Online doctors for NORDIMET 10 mg Injectable Solution in Pre-filled Pen
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for NORDIMET 10 mg Injectable Solution in Pre-filled Pen – subject to medical assessment and local rules.