
NITROFIX 10 mg TRANSDERMAL PATCHES


How to use NITROFIX 10 mg TRANSDERMAL PATCHES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the user
NITROFIX 10 mg transdermal patches EFG
Nitroglycerin
Read this leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is NITROFIX 10 mg and what is it used for
- What you need to know before taking NITROFIX 10 mg
- How to use NITROFIX 10 mg
- Possible side effects
- Storage of NITROFIX 10 mg
- Package contents and additional information
1. What is NITROFIX 10 mg and what is it used for
NITROFIX 10 mg patches are transdermal systems consisting of a thin, transparent polyethylene film of low density, covered by an adhesive matrix containing nitroglycerin. The matrix controls the release rate of the active ingredient. The patch is coated with a protective polyester film that is removed and discarded before use.
NITROFIX 10 mg is indicated for the prevention of effort and rest angina associated with coronary insufficiency.
2. What you need to know before taking NITROFIX 10 mg
Do not use NITROFIX 10 mg
- If you are allergic (hypersensitive) to organic nitrates or any other component of NITROFIX 10 mg.
- If you have severe anemia, cerebral hemorrhage, or cranioencephalic trauma with increased intracranial or intraocular pressure (glaucoma).
- In case of acute circulatory failure (shock, collapse states).
- If you are being treated with medications containing sildenafil. Patients being treated with this medication should never take medications containing sildenafil (medication used for erectile dysfunction) concurrently. For more information, consult your doctor or pharmacist.
Be careful with NITROFIX 10 mg
NITROFIX 10 mg should be used under strict medical supervision in cases of myocardial infarction or congestive heart failure. Caution is recommended when using NITROFIX 10 mg in patients with hypoxemia or ventilation-perfusion imbalance.
NITROFIX 10 mg is not indicated for the treatment of acute angina attacks. In this case, your doctor will prescribe rapid-acting nitro derivatives for sublingual use.
If treatment is to be discontinued, the dose and frequency of NITROFIX 10 mg patch application should be gradually reduced over a period of 4-6 weeks to avoid withdrawal reactions from this class of vasodilator drugs.
This medication may cause postural hypotension, especially in anxious patients, so avoid sudden changes in posture.
It is recommended to remove the patch before cardioversion or defibrillation.
Tolerance to the preparation or other nitro derivatives may occur. For this reason, your doctor may recommend applying NITROFIX 10 mg daily with a patch-free interval of 8 to 12 hours to maintain low plasma levels.
Nitroglycerin may interfere with the measurement of certain clinical analyses (catecholamines and vanilmandelic acid in urine, increasing their excretion).
Immediately inform your doctor if any of the following situations occur while using NITROFIX 10 mg. Your doctor will decide whether it is necessary to interrupt treatment with NITROFIX 10 mg.
Use of other medications
Inform your doctor or pharmacist if you are using or have recently used other medications, including those purchased without a prescription.
It is advisable for your doctor to know if you are being treated with other medications, as they may enhance the blood pressure-lowering effect of NITROFIX 10 mg (calcium antagonists, beta-blockers, diuretics, antihypertensives, tricyclic antidepressants, major tranquilizers, and dihydroergotamine).
Administration with other vasodilators should be done with caution to avoid adding effects.
The possibility that the ingestion of acetylsalicylic acid and non-steroidal anti-inflammatory substances may decrease the therapeutic response to NITROFIX 10 mg cannot be excluded.
The effect of this medication on the heart may be altered if used concurrently with preparations containing sildenafil for penile erection (see contraindications).
The use of topical products, especially if prolonged, may cause sensitization phenomena. In this case, treatment should be discontinued, and appropriate therapeutic measures should be taken.
Use of NITROFIX 10 mg with food and beverages
Excessive alcohol consumption should be avoided.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medication.
NITROFIX 10 mg should be used with caution during pregnancy and breastfeeding. In these circumstances, the product is only suitable in case of need and under direct medical supervision. If you are pregnant, notify your doctor immediately if you are regularly using this medication.
Use in children:Safety and efficacy in children under 18 years have not been established. Therefore, its use is not recommended.
Use in elderly:Geriatric patients are often more sensitive to hypotensive effects.
Driving and using machines
Due to the potential side effects (dizziness, hypotension, etc.) that may decrease reaction capacity, maximum precautions should be taken when driving vehicles or operating machinery at the start of treatment.
3. How to use NITROFIX 10 mg
Follow your doctor's instructions for administering NITROFIX 10 mg exactly. Consult your doctor or pharmacist if you have any doubts.
The response to nitro derivatives varies from person to person, and the minimum effective dose should be prescribed in each case. Since the amount of nitroglycerin released by the NITROFIX 10 mg patch is constant, the administered dose depends solely on the patch's contact area.
The normal dose is to start treatment with a NITROFIX 5 mg patch per day. If necessary, and depending on the demonstrated tolerance, treatment can be increased to a NITROFIX 10 mg or even NITROFIX 15 mg patch per day.
Application can be for a continuous period of 24 hours or intermittently, incorporating a patch-free interval of 8 to 12 hours (usually at night).
INSTRUCTIONS FOR CORRECT ADMINISTRATION
Each NITROFIX 10 mg patch is contained in a small sealed protective bag. The adhesive area is coated with a protective film that should be removed just before application to the skin.
Apply the NITROFIX 10 mg patch to the skin in a clean, dry, healthy area (without cream residue) and with little hair.
The correct application procedure is as follows:
1. Open the bag by tearing it at the mark and remove the patch from the inside.
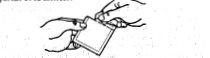
2. Fold the patch slightly with the dotted marks facing you, pull the tab, and discard the protective film.

3. Apply the adhesive surface to the upper arm or chest. Carefully separate the other dotted part of the protective film.

4. Press on the patch, ensuring its good placement.
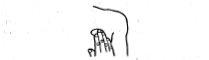
Remove the NITROFIX 10 mg patch after 24 hours, unless otherwise recommended by your doctor. NITROFIX 10 mg patches are disposable. They should be kept out of the reach of children.
Apply a new NITROFIX 10 mg patch following the method described. This new patch should be applied to a different area than the previous one, e.g., on the opposite side of the chest.
Do not apply the system to the same area for consecutive days. The NITROFIX 10 mg patch adheres easily to the skin and does not come off with bathing, showering, or physical exercise.
If you use more NITROFIX 10 mg than you should
High doses of nitroglycerin can, in some cases, induce a rapid decrease in blood pressure and cause shock, tachycardia, methemoglobinemia, cyanosis, coma, and convulsions. Due to the controlled release of nitroglycerin with NITROFIX 10 mg, the risk of overdose is very rare. Any reduction in blood pressure and symptoms of collapse can be treated by elevating the lower limbs. Severe methemoglobinemia can be treated with an injection of methylene blue or toluidine blue.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone: (91) 562 04 20.
If you forget to use NITROFIX 10 mg
Do not take a double dose to make up for forgotten doses. Ensure that you complete the treatment cycle.
If you interrupt treatment with NITROFIX 10 mg
If you have any other questions about using this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, NITROFIX 10 mg can cause side effects, although not everyone will experience them.
Tolerance to nitroglycerin is generally good. Headache is the most frequent side effect, especially when high doses are used. This effect is usually counteracted with common analgesics, although in particularly intense cases, it may be necessary to reduce the dose or interrupt treatment.
Other possible side effects that may appear, especially at the start of treatment, are: arterial hypotension (especially postural), weakness, tachycardia, fainting, palpitations, flushing, dizziness, nausea, vomiting, and dermatitis. Except for dermatitis, all of these are attributable to the pharmacological action of nitroglycerin. The doctor should be immediately informed of side effects, and this will give them the opportunity to suspend treatment with NITROFIX 10 mg, at least temporarily.
Local tolerance is also generally good. Occasionally, itching, burning, and mild redness reactions may occur, always depending on the level of application. However, these effects usually disappear a few hours after removing the patch without the need for other measures.
If you consider that any of the side effects you are experiencing is serious or if you notice any side effect not mentioned in this leaflet, inform your doctor or pharmacist.
5. Storage of NITROFIX 10 mg
No special storage conditions are required
Keep out of the reach and sight of children.
Do not use NITROFIX 10 mg after the expiration date stated on the packaging or carton, after "EXP". The expiration date is the last day of the month indicated.
Do not use NITROFIX 10 mg if you notice visible signs of deterioration.
Medications should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Composition of NITROFIX 10 mg
The active ingredient is Nitroglycerin 53 mg, releasing 10 mg/24 hours.
The other components are DURO-tak 872852, sorbitan monooleate, propylene glycol, low-density polyethylene oval film, and transparent polyester film.
Appearance of NITROFIX 10 mg and package contents
NITROFIX are transparent transdermal patches with a surface area of 13.3 cm2 and are presented in packages of 7 or 30 transdermal patches.
Marketing authorization holder and manufacturer
Marketing authorization holder:
ARAFARMA GROUP, S.A.
C/ Fray Gabriel de San Antonio, 6-10
Pol. Ind. del Henares
19180 Marchamalo (Guadalajara), Spain.
Manufacturer:
IBSA FARMACEUTICI ITALIA SRL
Strada Statale Nº 11, Padana Superiore, km 160
20051-Cassina de’ Pecchi (MI)
Italy
Or
ALTERGON ITALIA SRL
Zona Industriale ASI,
Morra de Sanctis - 83040 (Av)
Italy
This leaflet was approved in April 2019.
- Country of registration
- Average pharmacy price15.42 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NITROFIX 10 mg TRANSDERMAL PATCHESDosage form: TRANSDERMAL PATCH, 37.4 mg nitroglycerinActive substance: glyceryl trinitrateManufacturer: Merus Labs Luxco Ii S.À.R.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 18.7 mg nitroglycerinActive substance: glyceryl trinitrateManufacturer: Merus Labs Luxco Ii S.À.R.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 31.37 mgActive substance: glyceryl trinitrateManufacturer: Casen Recordati S.L.Prescription required
Online doctors for NITROFIX 10 mg TRANSDERMAL PATCHES
Discuss questions about NITROFIX 10 mg TRANSDERMAL PATCHES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











