
NICOTINELL COOL MINT 4 mg MEDICINAL CHEWING GUM

How to use NICOTINELL COOL MINT 4 mg MEDICINAL CHEWING GUM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Nicotinell Cool Mint 4 mg Medicinal Chewing Gum
Nicotine
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
This medication can be obtained without a prescription. However, to achieve the best results,
you should use Nicotinell Cool Mint 4 mg medicinal chewing gum correctly.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What is Nicotinell Cool Mint and what is it used for
- What you need to know before taking Nicotinell Cool Mint
- How to take Nicotinell Cool Mint
- Possible side effects
- Storage of Nicotinell Cool Mint
- Contents of the pack and further information
1. What is Nicotinell Cool Mint and what is it used for
Nicotinell belongs to a group of medications used as an aid to stop smoking.
Nicotinell contains the active ingredient nicotine.
When you chew the gum, the nicotine is released slowly and absorbed through the mouth mucosa.
It is indicated to relieve the symptoms of nicotine withdrawal syndrome in nicotine dependence, as an aid to stop smoking.
Counseling and patient support usually improve the chances of success.
2. What you need to know before taking Nicotinell Cool Mint
Do not take Nicotinell Cool Mint
- If you are allergic (hypersensitive) to nicotine or any of the other components of this medication (listed in section 6).
- If you are a non-smoker.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Nicotinell if you have:
- any heart problems, you should consult a healthcare professional before using any nicotine replacement product. If you experience an increase in heart problems while using a nicotine replacement therapy product, you should reduce or stop using the product,
- recently had a heart attack or stroke, or suffer from serious heart rhythm problems or chest pain, you should try to stop smoking without using any nicotine replacement therapy product, unless your doctor tells you to use it,
- heart failure, angina, Prinzmetal's angina, or high blood pressure (uncontrolled hypertension),
- circulation problems,
- diabetes, you should monitor your blood sugar levels more often when you start using nicotine gum. Your need for insulin or medication may change,
- overactive thyroid gland (hyperthyroidism),
- overactive adrenal glands (pheochromocytoma),
- suffer from kidney or liver failure,
- stomach or duodenal ulcers or inflammation of the esophagus or throat (the passage between the mouth and stomach), as nicotine replacement therapy may worsen your symptoms,
- fructose intolerance,
- if you have ever had seizures.
Keep all medications out of the sight and reach of children.
People with jaw joint problems and some people with dentures may have difficulty chewing the gum. If this is your case, it is recommended to use a different pharmaceutical form of nicotine replacement therapy. Consult your doctor or pharmacist.
Children and adolescents
Even small amounts of nicotine in children are dangerous and can cause serious symptoms or death. If poisoning is suspected in a child, consult a healthcare professional immediately. For this reason, it is essential to keep Nicotinell out of the sight and reach of children at all times.
Taking Nicotinell Cool Mint with other medications
Tell your doctor or pharmacist if you are taking, have recently taken, or may take any other medication. Stopping smoking can alter the effect of other medications you may be taking. If you have any questions or concerns about this, consult a healthcare professional.
There is no information about interactions between Nicotinell and other medications. However, apart from nicotine, other substances in cigarettes can affect some treatments.
Stopping smoking can change the effect of medications such as:
- Theophylline (used in the treatment of bronchial asthma).
- Tacrine (used in the treatment of Alzheimer's disease).
- Olanzapine and Clozapine (for the treatment of schizophrenia).
- Insulin (used in the treatment of diabetes), which may need to be adjusted.
Taking Nicotinell Cool Mint with food and drinks
Consuming coffee, acidic beverages (e.g., fruit juices), or soft drinks can decrease nicotine absorption, so they should be avoided during the 15 minutes prior to chewing Nicotinell, as they can affect nicotine absorption.
Pregnancy and breastfeeding
Pregnancy
If you are pregnant, you should stop smoking without using nicotine replacement therapy. However, if you have tried and failed, nicotine replacement therapy should only be used to help you stop smoking after the recommendation of a healthcare professional.
Breastfeeding
If you are breastfeeding, nicotine replacement therapy should only be used after the advice of a healthcare professional, as nicotine can pass into breast milk.
Fertility
Smoking increases the risk of infertility in women and men.
Driving and using machines
Although there are no known effects on the ability to drive or use machines at the recommended doses, it should be taken into account that stopping smoking causes changes in behavior.
Nicotinell Cool Mint contains sorbitol, butylhydroxytoluene, and sodium
This medication contains sorbitol, which is a source of fructose. If your doctor has told you that you have an intolerance to certain sugars, or if you have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disorder in which the person cannot break down fructose, consult with them before taking this medication. If you do not tolerate sorbitol, do not use this product.
This medication may cause local skin reactions (such as contact dermatitis) or irritation of the eyes and mucous membranes because it contains butylhydroxytoluene.
Each Nicotinell Cool Mint 4 mg gum contains 0.1 g of sorbitol, as a sweetener, which is a source of 0.02 g of fructose. The caloric value is 1.2 kcal/gum.
This medication contains less than 23 mg of sodium (1 mmol) per medicinal gum; that is, it is essentially "sodium-free".
3. How to take Nicotinell Cool Mint
Follow the administration instructions for Nicotinell contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
To improve the chances of success in stopping smoking, you should not smoke at all when starting to use Nicotinell, nor during the entire treatment.
Nicotinell is available in two dosages: 2 and 4 mg. The appropriate dose depends on your previous smoking habits. You should take Nicotinell 4 mg if:
- You are a smoker with high or very high nicotine dependence
- You have not been able to stop smoking previously using Nicotinell 2 mg
- Your symptoms from stopping smoking are so intense that you relapse and start smoking again
In any other case, you should use Nicotinell 2 mg.
Select the appropriate dosage for you based on the following table:
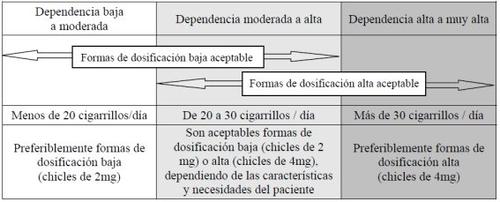
If you experience any adverse event when starting treatment with a high-dosage form (Nicotinell 4 mg), it should be replaced with a lower-dosage form (Nicotinell 2 mg).
Method of administration:
Start using this product on the day you stop smoking. To stop smoking successfully with this treatment, you must stop smoking completely.
Chew 1 gum when you feel the urge to smoke. Do not use more than 1 gum at a time. Do not use more than 1 gum per hour.
- Chew a gum slowly until the flavor becomes intense. Do not eat or drink while you have a gum in your mouth.
- Place the gum between your cheek and gum.
- When the flavor decreases, chew the gum again.
- Repeat this process for 30 minutes to release the nicotine gradually.
Medicinal gums should not be swallowed.
Adults over 18 years:
Chew a gum when you start to feel the need to smoke. In general, you should chew a gum every 1-2 hours. In most cases, 8-12 gums per day are sufficient. If you still feel the need to smoke, you can chew an extra gum. Do not take more than 15 Nicotinell 4 mg gums per day.
The duration of treatment is individual. Normally, treatment should be followed for 3 months. After 3 months, the number of gums per day will be gradually reduced. The treatment should end when the consumption of gums has been reduced to 1 or 2 gums per day.
It is not recommended to use Nicotinell for more than 6 months. However, some ex-smokers may need longer treatment with medicinal gums to avoid relapse.
If you are still using Nicotinell after 6 months, you should consult your doctor or pharmacist.
Counseling and patient support usually improve the chances of success.
Use in children and adolescents
Nicotinell should notbe used by minors under 18 years without the recommendation of a doctor.
If you take more Nicotinell Cool Mint than you should
Chewing too many Nicotinell gums can cause symptoms similar to those that occur when smoking a large amount of tobacco. If you use too many nicotine gums, you may start to feel sick, dizzy, and unwell. Stop using the gums and consult a healthcare professional immediately. General symptoms of nicotine overdose include: weakness, pale skin, increased sweating, involuntary muscle contractions, increased salivation, dizziness, throat burning, nausea, vomiting, diarrhea, abdominal pain, changes in vision and hearing, headache, alteration of heart rhythm (tachycardia), shortness of breath, confusion, prostration, circulation problems, coma, and terminal convulsions.
With very high overdoses, exhaustion, convulsions, low blood pressure leading to circulatory collapse, or respiratory failure may occur.
Consult your doctor or pharmacist if you experience any problems.
If poisoning is suspected in a child, you should consult a doctor immediately. Even small amounts of nicotine are dangerous and potentially fatal for children and can cause serious symptoms or death.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
If you forget to use Nicotinell
Do not take a double dose to make up for the forgotten dose.
If you stop using Nicotinell
If you have more questions about the use of this medication, consult your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can have side effects, although not everyone will experience them.
Some of the effects you may notice during the first few days are dizziness, headache, and sleep disturbances, which can be attributed to the symptoms related to stopping smoking, and sometimes may be due to insufficient nicotine administration.
They are grouped according to the likelihood with which they may occur:
Very common(may affect 1 in 10 patients)
- feeling unwell (nausea).
Common(may affect 1 in 100 patients):
- the gums may cause mild pain or irritation in the mouth or tongue
- sore throat or inflammation
- feeling sick
- stomach upset
- diarrhea
- indigestion/heartburn
- flatulence
- hiccups
- excessive salivation
- constipation
- difficulty swallowing
- dizziness and headache
- vomiting
- insomnia
- cough
- dry mouth
- and jaw muscle pain, especially as a result of intense gum chewing. Chewing the gum more slowly can solve these problems.
Uncommon(may affect 1 in 1,000 patients):
- feeling heartbeats (palpitations)
- itching of skin bumps (urticaria).
Rare(may affect 1 in 10,000 patients):
- rapid or irregular heartbeats
- difficulty breathing
- symptoms of a severe allergic reaction, including sudden wheezing or chest tightness, skin rash, and feeling of fainting
- heart rhythm disturbances (faster heartbeat) and allergic reactions. Allergic reactions can, in very rare cases, be severe and have the following symptoms:
- skin swelling;
- facial and mouth inflammation;
- dizziness, dizziness, and fainting (symptoms of low blood pressure);
- difficulty breathing.
If you experience any of these symptoms, STOP taking Nicotinell medicinal gum and seek medical help immediately.
Mouth ulcers may occur, which may be related to stopping smoking, not to the treatment.
In rare cases, Nicotinell may adhere to and damage dentures or other dental prostheses.
If you consider that any of the side effects you are experiencing is serious or if you notice any other side effect not mentioned in this package leaflet, inform your doctor or pharmacist.
Reporting of side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use. Website: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Nicotinell Cool Mint
Keep this medication out of the sight and reach of children.
Do not use Nicotinell after the expiration date stated on the packaging after CAD. The expiration date is the last day of the month indicated.
Do not store above 25°C.
Do not throw away medications through wastewater or household waste. Deposit the packaging and medications you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Packaging Content and Additional Information
Nicotinell Cool Mint Composition
- The active ingredient is nicotine. Each medicinal chewing gum contains 4 mg (corresponding to 20 mg of nicotine-polacrilin).
- The other components are: sorbitol (E420), gum base (contains butylhydroxytoluene (E321)), calcium carbonate, anhydrous sodium carbonate, sodium hydrogen carbonate, polacrilin, glycerol (E422), purified water, levomenthol, natural peppermint flavor, peppermint millicaps, sucralose, potassium acesulfame, xylitol (E967), mannitol (E421), gelatin, titanium dioxide (E171), carnauba wax, and talc. See section 2 "Nicotinell Cool Mint contains sorbitol, butylhydroxytoluene, and sodium".
Product Appearance and Packaging Content
Nicotinell medicinal chewing gum is available in two doses (2 and 4 mg) and three flavors (Nicotine, Fruit, and Cool Mint). This leaflet corresponds to Nicotinell Cool Mint 4 mg medicinal chewing gum.
Nicotinell 4 mg is presented in the form of a white, matte medicinal chewing gum with a rectangular shape.
Nicotinell 4 mg is available in packs of 2, 12, 24, 36, 48, 60, 72, 96, 120, or 204 medicinal chewing gums in blister packs. Not all pack sizes may be marketed.
Marketing Authorization Holder
Haleon Spain, S.A.
Parque Tecnológico de Madrid, Calle de Severo Ochoa, 2
28760 Tres Cantos, Madrid – Spain
Manufacturer
FAMAR S.A.
48th km National Road Athens-Lamia,
19011, Avlonas, Attiki – Greece
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany Nicotinell Kaugummi 4 mg Cool Mint
Austria Nicotinell MintFrisch 4 mg - Kaugummi
Belgium Nicotinell Cool Mint, 4 mg, kauwgom
Denmark Nicotinell Mint
Spain Nicotinell Cool Mint 4 mg medicinal chewing gum
Estonia Nicotinell Cool Mint
Greece Nicotinell Cool Mint 4 mg medicated chewing-gum
Finland Nicotinell Mint 4 mg lääkepurukumi
France NICOTINELL MENTHE FRAICHEUR 4 mg SANS SUCRE, gomme à mâcher médicamenteuse
Ireland Nicotinell Cool Mint 4mg Medicated Chewing Gum
Iceland Nicotinell Mint 4 mg lyfjatyggigúmmí
Lithuania Nicotinell Mint 4 mg medicated chewing-gum
Luxembourg Nicotinell Cool Mint, 4 mg, gomme à mâcher médicamenteuse
Latvia Nicotinell Cool Mint 4 mg medicated chewing gum
Norway Nicotinell medisinsk tyggegummi med peppermyntesmak
Netherlands Nicotinell Cool Mint, 4 mg, kauwgom
Portugal Nicotinell Freshmint 4 mg
United Kingdom Nicotinell Ice Mint 4mg Medicated Chewing Gum
Sweden Nicotinell Mint 4 mg
This leaflet was last revised in November 2021
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NICOTINELL COOL MINT 4 mg MEDICINAL CHEWING GUMDosage form: CHEWING GUM, 2 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not requiredDosage form: CHEWING GUM, 4 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not requiredDosage form: CHEWING GUM, 2 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not required
Online doctors for NICOTINELL COOL MINT 4 mg MEDICINAL CHEWING GUM
Discuss questions about NICOTINELL COOL MINT 4 mg MEDICINAL CHEWING GUM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













