NEUPRO 3 mg/24h TRANSDERMAL PATCH

How to use NEUPRO 3 mg/24h TRANSDERMAL PATCH
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Neupro 1 mg/24 h Transdermal Patch
Neupro 3 mg/24 h Transdermal Patch
Ropinirole
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is Neupro and what is it used for
- What you need to know before you use Neupro
- How to use Neupro
- Possible side effects
- Storing Neupro
- Contents of the pack and further information
1. What is Neupro and what is it used for
What is Neupro
Neupro contains the active substance ropinirole.
It belongs to a group of medicines known as “dopamine agonists”. Dopamine is an important messenger in the brain for movement.
What is Neupro used for
Neupro is used in adults to treat the signs and symptoms of:
- Restless Legs Syndrome(RLS) – this syndrome may be associated with discomfort in the legs or arms, a need to move, sleep disturbances, and a feeling of tiredness or sleepiness during the day. Treatment with Neupro reduces or decreases the duration of these symptoms.
2. What you need to know before you use Neupro
Do not use Neupro if:
- you are allergicto ropiniroleor any of the other ingredientsof this medicine (listed in section 6)
- you are going to have an MRI scan(magnetic resonance imaging, a diagnostic test that uses magnetic energy instead of X-rays)
- you need cardioversion(a specific treatment for heart rhythm disturbances).
You must remove the Neupro patch just before having an MRI scan or cardioversion to avoid skin burns because the patch contains aluminum. You can put on a new patch when these tests are over.
Do not use Neupro if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist first.
Warnings and precautions
Talk to your doctor or pharmacist before you start using Neupro, as:
- you should have your blood pressurechecked regularly while you are using Neupro, especially when you start treatment. Neupro may affect your blood pressure.
- you should have your eyeschecked regularly while you are using Neupro. If you notice any problems with your eyes between checks, tell your doctor immediately.
- if you have severe liver problems, your doctor may need to adjust your dose. If your liver problems get worse while you are using Neupro, tell your doctor as soon as possible.
- you may have skin reactionscaused by the patch – see ‘Skin problems caused by the patch’ in section 4.
- you may feel very sleepy or fall asleep suddenly– see ‘Driving and using machines’ in section 2.
- the symptoms of Restless Legs Syndromemay start earlier than usual, be more intense, and involve other parts of the body. If you experience these symptoms before or after starting treatment with Neupro, contact your doctor as you may need to have your treatment adjusted.
You may lose consciousness
Neupro may cause loss of consciousness. This can happen especially when you start treatment with Neupro or when you increase the dose. Tell your doctor if you lose consciousness or feel dizzy.
Changes in behavior and abnormal thoughts
Neupro may cause side effects that change your behavior (how you act). If your family or caregiver, or your doctor, are concerned about changes in your behavior, it may be helpful to tell a family member or caregiver that you are using this medicine and for them to read this leaflet.
For more information, see ‘Changes in behavior and abnormal thoughts’ in section 4.
Children and adolescents
This medicine must notbe given to childrenunder 18 years because its safety and effectiveness in this age group are not known.
Using Neupro with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes medicines that you buy without a prescription and herbal medicines.
Do not take the following medicines while you are using Neupro, as they may reduce its effect:
- ‘antipsychotic’ medicines - used to treat certain mental illnesses
- metoclopramide - used to treat nausea and vomiting.
Talk to your doctor before using Neupro if you are taking:
- sedative medicines such as benzodiazepines or medicines used to treat mental disorders or depression
- medicines that lower blood pressure. Neupro may lower blood pressure when you stand up - this effect could be made worse by taking medicines to lower blood pressure.
Your doctor will tell you if it is safe to take these medicines while you are using Neupro.
Using Neupro with food, drinks, and alcohol
Since ropinirole enters the bloodstream through the skin, taking food or drinks does not affect how this medicine is absorbed. You should talk to your doctor if you can drink alcohol while using Neupro.
Pregnancy and breastfeeding
Do not use Neupro if you are pregnant. This is because the effects of ropinirole on pregnancy and the unborn child are not known.
Do not breastfeed while you are using Neupro. This is because ropinirole may pass into breast milk and affect your baby. It may also reduce the amount of milk produced.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Neupro may make you feel very sleepy or fall asleep suddenly. If this happens, do not drive.
In rare cases, some people have fallen asleep while driving when using Neupro.
Do not use tools or machines if you feel very sleepy - or do any other activity that could put you or others at risk of serious harm.
Neupro contains sodium metabisulfite (E223)
Neupro contains sodium metabisulfite (E223), a substance that can rarely cause severe allergic reactions and bronchospasm (difficulty breathing due to narrowing of the airways).
3. How to use Neupro
Follow the instructions for using this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
What patch dose to use
Neupro is available in patches of different strengths that release the medicine over 24 hours. The strengths are 1 mg/24 h, 2 mg/24 h, and 3 mg/24 h for the treatment of Restless Legs Syndrome.
- Your starting dose will be a 1 mg/24 h patch per day.
- From the second week onwards, your daily dose will be increased by 1 mg weekly until you reach the right maintenance dose for you. This is when you and your doctor confirm that your symptoms are adequately controlled and that the side effects of the medicine are acceptable.
- Follow your doctor’s instructions carefully.
- The maximum dose is 3 mg per day.
If you have to stop using this medicine, see “If you stop using Neupro” in section 3.
How to use the Neupro patches
Neupro is a patch that you apply to your skin.
- Check that you have removed the used patch before applying a new one.
- Apply the new patch to a different area of skin each day.
- Leave the patch on your skin for 24 hours, then remove it and apply a new one.
- Change the patchat about the same time every day.
- Do not cut the Neupro patches into pieces.
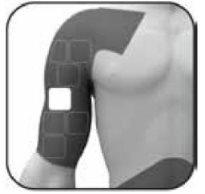
Where to apply the patch Apply the adhesive side of the patch to clean, dry, and healthy skin on the following areas, as indicated by the grey areas in the diagram:
|
|
To avoid skin irritation
|
|
If you continue to have skin problems caused by the patch, see “Skin problems caused by the patch” in section 4 for more information.
To prevent the patch from coming loose or falling off
- Do notapply the patch to an area where it may be rubbed by tight clothing.
- Do notuse creams, oils, lotions, powders, or any other skin productswhere you are going to apply the patch. Nor on top of or near another patch that you are already using.
- If you need to apply a patch to an area of skin where you have hair, you should shave(remove hair from) the area of skin at least 3 days beforeyou apply the patch to that site.
- If the edges of the patch come loose, you can secure the patch with a band-aid.
If the patch falls off, you should apply a new one for the rest of the day and the next day, apply a new patch at the usual time.
- You should avoidheating the patch area- for example, too much sunlight, saunas, hot baths, hot water bottles, or heat pads. This is because the medicine could be released more quickly. If you think you have overheated, contact your doctor or pharmacist.
- Always check that the patch has not fallen off after activities such as bathing, showering, or exercising.
- If the patch has irritated your skin, you should keepthe irritated area protected from direct sunlight. This is because sun exposure could cause changes in skin color.
How to use the patch
- Each patch is packaged individually in a pouch.
- Before you open the pouch, decide where you are going to apply the new patch and check that you have removed the used patch.
- When you have opened the pouch and removed the disposable liner from the patch, you should apply the patch to your skin immediately.
1. To open the pouch, hold both sides of the pouch with both hands. |
|
2. Peel off the liners. |
|
3. Open the pouch. |
|
4. Remove the patch from the pouch. |
|
5. The adhesive side of the patch is covered by a transparent disposable liner.
|
|
6.
|
|
7.
|
|
8.
|
|
9. Fold back the other half of the patch and remove the rest of the disposable liner. |
|
10.
This ensures that the patch is in contact with your skin and the edges are well stuck. |
|
11. Wash your hands with soap and water immediately after handling the patch. |
How to remove the used patch
- Remove the used patch slowly and carefully.
- Gently wash the area with warm water and a mild soap. This will remove any remaining adhesive from your skin. You can also use a little baby oil to remove any remaining adhesive.
- Do not use alcohol or other liquid solvents - such as nail varnish remover. This could irritate your skin.
If you use more Neupro than you should
Using higher doses of Neupro than your doctor has prescribed may cause side effects such as nausea or vomiting, low blood pressure, seeing or hearing things that are not real (hallucinations), confusion, extreme sleepiness, involuntary movements, and convulsions.
In these cases, tell your doctor or go to the hospital as soon as possible. They will tell you what to do.
If you forget to change the patch at the usual time
- If you have forgotten to change the patch at the usual time, change it as soon as you remember. Remove the used patch and apply a new one.
- If you have forgotten to apply a new patch after removing the used patch, apply a new one as soon as you remember.
In both cases, the next day, apply a new patch at the usual time. Do not use a double dose to make up for forgotten doses.
If you stop using Neupro
Do not stop using Neupro without talking to your doctor first. Stopping Neupro suddenly may cause you to have a condition called ‘neuroleptic malignant syndrome’ which can be life-threatening. The signs include: loss of muscle movement (akinesia), muscle stiffness, fever, unstable blood pressure, increased heart rate (tachycardia), confusion, decreased consciousness (for example, coma).
If your doctor tells you to stop using Neupro, the daily doseof Neupro should be gradually reduced:
- 1 mg every 2 days – if you are using Neupro for Restless Legs Syndrome
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Inform your doctor or pharmacist if you notice any adverse effect.
Most Likely Adverse Effects at the Start of Treatment
You may experience nauseaand vomiting at the start of treatment. These effects are usually mild or moderate and last for a short time. You should consult your doctorif the effects last for a long time or if you are concerned.
Skin Problems Caused by the Patch
- You may experience redness and itching in the area of the skin where the patch has been applied – these reactions are usually mild or moderate.
- These reactions usually disappear a few hours after removing the patch.
- Consult your doctorif you have a skin reaction that lasts for more than a few days, if it is severe, or if it spreads beyond the area covered by the patch.
- Avoid exposure to the sun and sunlamps in areas of skin that show any reaction caused by the patch.
- To help prevent skin reactions, you should apply the patch to a different site each day and only reuse the same area after 14 days.
You May Experience Loss of Consciousness
Neupro can cause loss of consciousness. This can happen especially when starting treatment with Neupro or when increasing the dose. Inform your doctor if you lose consciousness or feel dizzy.
Changes in Behavior and Abnormal Thoughts
Inform your doctor if you notice any change in behavior, thought, or both, as indicated below.
Your doctor will indicate how to manage or reduce the symptoms.
If your family or caregiver, or your doctor, are concerned about changes in your behavior, it may be helpful to tell a family member or caregiver that you are using this medicine and to read the package insert. Neupro can cause anxiety or an overwhelming urge to behave abnormally and be unable to control the impulse, attack, or temptation to perform certain actions that may harm you or others.
These actions may include:
- strong addiction to gambling – even seriously affecting you or your family
- altered or increased sexual interest and behavior that causes great concern for you and others - for example, increased sexual desire
- uncontrolled shopping or excessive spending
- episodes of binge eating (eating large amounts of food in a short period) or compulsive eating (eating more food than normal or more than needed to satisfy your appetite)
Neupro can cause other abnormal behaviors and thoughts, which may include:
- abnormal thoughts about reality
- delusional ideas and hallucinations (seeing or hearing things that are not real)
- confusion
- disorientation
- aggressive behavior
- agitation
- delirium
Inform your doctor if you notice any change in your behavior, thought, or both, as indicated above.
Your doctor will indicate how to manage or reduce the symptoms.
Allergic Reactions
Inform your doctor if you notice signs of an allergic reaction – which may include swelling of the face, tongue, or lips.
Adverse Effects if You Use Neupro for Restless Legs Syndrome
Inform your doctor or pharmacist if you experience any of the following adverse effects:
Very Common: may affect more than 1 in 10 patients
- headache
- nausea
- feeling of weakness (fatigue)
- skin irritation at the patch application site, such as redness and itching
Common: may affect up to 1 in 10 patients
- itching
- feeling of irritability
- allergic reaction
- increased sexual desire
- increased blood pressure
- vomiting, heartburn
- swelling in the legs and feet
- drowsiness, sudden sleep, difficulty sleeping, sleep problems, unusual dreams
- inability to control the impulse to perform a harmful action, including addiction to gambling, repetitive actions, compulsive shopping, or excessive spending
- episodes of binge eating (eating large amounts of food in a short period), compulsive eating (eating more food than normal or more than needed to satisfy your appetite)
Uncommon: may affect up to 1 in 100 patients
- feeling agitated
- feeling dizzy when standing up due to low blood pressure
Rare: may affect up to 1 in 1,000 patients
- aggressive behavior
- disorientation
Not Known: frequency not known
- anxiety about taking high doses of medicines like Neupro – more than necessary for the treatment of the disease. This is known as 'dopaminergic dysregulation syndrome' and can lead to excessive use of Neupro.
- seeing or hearing things that are not real (hallucinations)
- nightmares
- paranoia
- confusion
- psychotic disorders
- delusional ideas
- delirium
- dizziness
- loss of consciousness, involuntary movements (dyskinesia)
- involuntary muscle spasms (convulsions)
- blurred vision
- visual disturbances such as seeing colors or lights
- vertigo (feeling of spinning)
- increased heart rate (palpitations)
- abnormal heart rhythm
- low blood pressure
- hypersensitivity
- constipation, dry mouth
- stomach discomfort and pain
- diarrhea
- redness, increased sweating
- generalized itching, skin irritation
- generalized rash
- inability to achieve or maintain an erection
- weight loss, weight gain
- abnormal liver test results or elevated
- increased heart rate
- increased levels of creatine phosphokinase (CPK) (CPK is an enzyme found mainly in skeletal muscle)
- falls
- rhabdomyolysis (a rare, severe muscle disorder that causes pain, sensitivity, and weakness of the muscles and can cause kidney problems)
Inform your doctor or pharmacist if you experience any of these adverse effects.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this package insert. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Neupro
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date shown on the label and carton.
Do not store above 30°C.
What to Do with Used and Unused Patches
Used patches still contain the active ingredient, 'rotigotine', which can be hazardous to others. Fold the used patch with the adhesive side inward. Place the patch in the original envelope and then throw it away in a safe place, out of the reach of children.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Neupro
The active ingredient is rotigotine.
- 1 mg/24 h:
A patch releases 1 mg of rotigotine every 24 hours. Each 5 cm2 patch contains 2.25 mg of rotigotine.
- 3 mg/24 h:
A patch releases 3 mg of rotigotine every 24 hours. Each 15 cm2 patch contains 6.75 mg of rotigotine.
The other ingredients are:
- Poly(dimethylsiloxane, trimethylsilyl silicate) copolymer, povidone K90, sodium metabisulfite (E223), ascorbyl palmitate (E304), and DL-α-tocopherol (E307).
- Covering layer: Polyester film, siliconized, aluminized, colored with a pigment layer (titanium dioxide (E171), pigment yellow 13, pigment red 166, pigment yellow 12) and printed (pigment red 146, pigment yellow 180, pigment black 7).
- Disposable coating: Transparent polyester film coated with fluoropolymer.
Appearance and Package Contents
Neupro is a transdermal patch. It is thin and has three layers. It is square in shape with rounded corners. The outer layer is brown in color and bears the inscription Neupro 1 mg/24 h or 3 mg/24 h.
Neupro is available in the following formats:
Cartons containing 7, 14, 28, 30, or 84 (multiple package containing 3 cartons of 28) patches, each patch is included in an individual envelope.
Not all pack sizes may be marketed.
Marketing Authorization Holder
UCB Pharma S.A.
Allée de la Recherche 60
B-1070 Brussels
Belgium
Manufacturer
UCB Pharma S.A.
Chemin du Foriest
B-1420 Braine l’Alleud
Belgium
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien UCB Pharma SA/NV Tel: +32-(0)2 559 92 00 | Lietuva UCB Pharma Oy Finland Tel: +358-92 514 4221 (Suomija) |
| Luxembourg/Luxemburg UCB Pharma SA/NV Tél/Tel: +32-(0)2 559 92 00 |
Ceská republika UCB s.r.o. Tel: +420-221 773 411 | Magyarország UCB Magyarország Kft. Tel.: +36-(1) 391 0060 |
Danmark UCB Nordic A/S Tlf.: +45-32 46 24 00 | Malta Pharmasud Ltd. Tel: +356-21 37 64 36 |
Deutschland UCB Pharma GmbH Tel: +49-(0)2173 48 48 48 | Nederland UCB Pharma B.V. Tel: +31-(0)76-573 11 40 |
Eesti UCB Pharma Oy Finland Tel: +358-92 514 4221 (Soome) | Norge UCB Nordic A/S Tlf: +45-32 46 24 00 |
Ελλ?δα UCB Α.Ε. Τηλ: +30-2109974000 | Österreich UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
España UCB Pharma S.A. Tel: +34-91 570 34 44 | Polska UCB Pharma Sp. z o.o. Tel.: +48-22 696 99 20 |
France UCB Pharma S.A. Tél: +33-(0)1 47 29 44 35 | Portugal BIAL-Portela & Cª, S.A. Tel: +351-22 986 61 00 |
Hrvatska Medis Adria d.o.o. Tel: +385-(0)1 230 34 46 | România UCB Pharma România S.R.L. Tel: +40-21 300 29 04 |
Ireland UCB (Pharma) Ireland Ltd. Tel: +353-(0)1-46 37 395 | Slovenija Medis, d.o.o. Tel: +386-1 589 69 00 |
Ísland Vistor hf. Sími: +354-535 7000 | Slovenská republika UCB s.r.o., organizačná zložka Tel: +421-(0)2 5920 2020 |
Italia UCB Pharma S.p.A. Tel: +39-02 300 791 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: +358-92 514 4221 |
Κ?προς Lifepharma (Z.A.M.) Ltd Τηλ: +357-22 05 63 00 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: +358-92 514 4221 |
Latvija UCB Pharma Oy Finland Tel: +358-92 514 4221 (Somija) |
Date of Last Revision of this Package Insert:
Other Sources of Information
Detailed information about this medicine is available on the European Medicines Agency website https://www.ema.europa.eu
- Country of registration
- Average pharmacy price48.71 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEUPRO 3 mg/24h TRANSDERMAL PATCHDosage form: TRANSDERMAL PATCH, 1 mg/24 hActive substance: rotigotineManufacturer: Exeltis Healthcare S.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 2 mg/24 hActive substance: rotigotineManufacturer: Exeltis Healthcare S.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 3 mg/24 hActive substance: rotigotineManufacturer: Exeltis Healthcare S.L.Prescription required
Online doctors for NEUPRO 3 mg/24h TRANSDERMAL PATCH
Discuss questions about NEUPRO 3 mg/24h TRANSDERMAL PATCH, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions