
NETDEX 3 mg/mL + 1 mg/mL Eye Drops in Single Dose Containers

How to use NETDEX 3 mg/mL + 1 mg/mL Eye Drops in Single Dose Containers
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
NETDEX 3mg/ml + 1mg/ml eye drops solution in single-dose container
netilmicin/dexamethasone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What NETDEX is and what it is used for
- What you need to know before you use NETDEX
- How to use NETDEX
- Possible side effects
- Storage of NETDEX
- Contents of the pack and other information
1. What NETDEX is and what it is used for
NETDEX contains two medicines: netilmicin and dexamethasone.
- Netilmicin is an antibiotic that eliminates bacteria.
- Dexamethasone is a corticosteroid that reduces inflammation.
Antibiotics are used to treat bacterial infections and do not work for viral infections. It is important that you follow the instructions regarding dose, administration interval, and treatment duration indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after finishing treatment, return it to the pharmacy for proper disposal. Do not throw medicines down the drain or in the trash. |
NETDEX is used in adults to reduce inflammation and eliminate bacteria in the eyes that are swollen, irritated, and likely to be infected with bacteria.
You should consult a doctor if your condition worsens or does not improve at the end of treatment.
2. What you need to know before you use NETDEX
Netilmicin/dexamethasone can be used in adults, including the elderly.
It is not recommended for people under 18 years of age.
Do not useNETDEX:
- If you are allergic to netilmicin, dexamethasone, antibiotics known as aminoglycoside antibiotics (such as tobramycin, kanamycin, amikacin, gentamicin, etc.) or to any of the other components of this medicine (listed in section 6).
- If your doctor has indicated that your eye pressure is too high.
- If you think you may have a viral or fungal infection in the eye or around the eye.
- If you have now, or have had in the past, a viral eye infection caused by the herpes simplex virus (HSV).
- If your doctor has warned you that you have an eye infection caused by bacteria known as mycobacteria.
Warnings and precautions
Consult your doctor or pharmacist before starting to use NETDEX.
Consult your doctor if you experience swelling and weight gain around the trunk and in the face, as these are usually the first signs of a syndrome called Cushing's syndrome. Suppression of adrenal gland function may occur after interrupting intensive or long-term treatment with netilmicin/dexamethasone. Consult your doctor before stopping treatment on your own. These risks are especially important in children and patients treated with a medicine called ritonavir or cobicistat.
Contact your doctor if you experience blurred vision or other visual disturbances.
If you useNETDEXfor a long time
- eye pressure may increase and cause damage to the optic nerves, leading to vision problems. If you use netilmicin/dexamethasone for more than 15 days, your doctor should check your eye pressure regularly;
- you may develop cataracts;
- wounds may take longer to heal;
- your body may not defend itself against other types of eye infections as well as it should, such as fungal or viral infections;
- eye infections that produce a lot of pus, with the use of corticosteroids, may worsen or it may be more difficult to identify the type of bacteria that cause the infection;
- the corticosteroid in netilmicin/dexamethasone may cause the surfaces of the eye to narrow and even perforate;
- you may start to become allergic to the antibiotic in the eye drops.
Before using NETDEX, inform your doctor if
- you have glaucoma or a family history of glaucoma;
- you have corneal problems;
- you are taking other medicines that contain phosphates. Your doctor will monitor your cornea at regular intervals.
Children and adolescents
NETDEX is not recommended for use in children or adolescents (from birth to 18 years of age).
For external use only
Use NETDEX only on the surface of the eye. This medicine should not be injected or swallowed.
Using NETDEX with other medicines
NETDEX may interact with other medicines. Inform your doctor or pharmacist if you are using, have recently used, or may need to use any eye product or any other medicine, including those obtained without a prescription. You can also use netilmicin/dexamethasone with other eye products, but you must follow the instructions in section 3.
Especially, inform your doctor or pharmacist if you are taking:
- any other antibiotic, in particular polymyxin B, colistin, viomycin, streptomycin, vancomycin, or cefaloridine. The simultaneous use of netilmicin/dexamethasone with other antibiotics may increase the risk of kidney problems, hearing problems, or may affect the proper functioning of the other antibiotics;
- cisplatin (an anticancer medicine);
- diuretics (medicines that reduce fluid retention) such as etacrynic acid and furosemide;
- anticholinergic medicines (medicines that stop gland secretion), such as atropine;
- ritonavir or cobicistat, as these may increase the amount of dexamethasone in the blood;
- other medicines that contain phosphates. Your doctor will monitor your cornea at regular intervals.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Use during pregnancy
It is preferable not to use NETDEX during pregnancy unless your doctor considers it necessary.
Use during breastfeeding
NETDEX should not be used if you are breastfeeding.
Driving and using machines
When using NETDEX, your vision may become blurred for a short time. If this happens, do not drive or use machines until your vision clears.
NETDEX contains phosphates
This medicine contains 0.18 mg of phosphates in each drop, equivalent to 3.66 mg/ml. If you have severe damage to the transparent layer at the front of the eye (the cornea), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation.
3. How to use NETDEX
Follow the administration instructions of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is one dropin the affected eye four times a dayor as prescribed. The normal treatment duration may vary from 5 to 14 days.
Do not change the dose of the eye drops without consulting your doctor.
Use in children and adolescents
NETDEX is not recommended for use in children or adolescents (from birth to 18 years of age).
Contact lens users
NETDEX in single-dose containers can be used while wearing contact lenses because it does not contain preservatives. However, it is strongly recommended to avoid wearing contact lenses during an eye infection or inflammation. Contact lenses should not be used during treatment with corticosteroid eye drops due to the increased risk of infection.
If you use NETDEX with other eye drops
Wait at least 10 minutes between using NETDEX and using other eye drops or ointments. Eye ointments should be used last.
Instructions for use
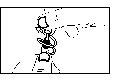
Make sure the single-dose container is intact.
- Open the aluminum pouch containing the single-dose containers.
- Remove a single-dose container from the strip (Drawing 1) and put the unopened containers back in the pouch.
- Open by twisting the top without pulling (Drawing 2). Do not touch the tip after opening the container.
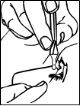
- Tilt your head back.
- With your finger, gently pull down the lower eyelid of the affected eye.
- Invert the single-dose container and place the tip of the container close to the eye, without touching it. Do not touch the eye or eyelid with the tip of the container (Drawing 3).
- Press the single-dose container to administer only one drop, and then release the lower eyelid.
- Close your eye and press with a finger on the corner of the affected eye, next to the nose. Hold for 2 minutes.
- Repeat in the other eye if your doctor has indicated it.
- Discard the single-dose container after use.

If you use the eye drops incorrectly, they may become contaminated with bacteria, which can lead to eye infections. Using contaminated eye drops can cause serious eye damage and subsequent vision loss.
If you use more NETDEX than you should
If you use too much eye drops, it is unlikely to cause problems.
Apply the next dose as usual.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken.
If you forget to use NETDEX
Do not use a double dose to make up for forgotten doses.
Apply the next dose as usual.
If you stop treatment with NETDEX
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency of the effect in each person cannot be estimated from the available data.
Eye disorders
Increased eye pressure, cataract formation after prolonged treatment, blurred vision, development or worsening of a viral eye infection caused by the herpes simplex virus (HSV) or a fungal infection, delayed healing of wounds.
In very rare cases (less than 1 in 10,000 people), some patients with severe damage to the transparent layer at the front of the eye (the cornea) have developed opaque spots in the cornea due to calcium accumulation during treatment.
Immune system disorders
Local allergic reaction: conjunctival redness, burning, itching.
Endocrine disorders
Excessive body hair growth (particularly in women), weakness and muscle wasting, purple striae on the skin of the body, increased blood pressure, irregular or absent menstrual periods, changes in body protein and calcium levels, delayed growth in children and adolescents, and swelling and weight gain of the body and face (called Cushing's syndrome) (see section 2 "Warnings and precautions").
In all the above cases, it is recommended to stop treatment.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of NETDEX
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, pouch, and container after "EXP". The expiry date is the last day of the month indicated.
Store below 30°C.
Keep the single-dose containers in the pouch to protect them from light and moisture.
This medicine does not contain preservatives.
After opening a single-dose container, use it immediately and discard the container with any remaining contents after use.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the SIGRE collection point at the pharmacy. If you have any further questions, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and other information
Composition of NETDEX
The active substances are dexamethasone 1 mg/ml (as disodium phosphate dexamethasone) and netilmicin 3 mg/ml (as netilmicin sulfate). Each single-dose container contains 0.9 mg of netilmicin and 0.3 mg of dexamethasone.
The other ingredients are:
sodium citrate,
sodium phosphate monobasic monohydrate,
disodium phosphate dodecahydrate,
purified water.
Appearance of the product and contents of the pack
NETDEX is a clear, colorless or slightly yellowish solution.
NETDEX eye drops in single-dose containers:
Five single-dose containers, each with 0.3 ml of NETDEX eye drops solution, wrapped in an aluminum pouch.
Each container contains 15 or 20 single-dose containers.
Only some pack sizes may be marketed.
Marketing authorization holder and manufacturer
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci Sant'Antonio (CT)
Italy
Local representative:
SIFI IBÉRICA S.L.
C/ Poeta Joan Maragall, 47
Planta 4, Puerta 402
28020 Madrid - Spain
Date of the last revision of this leaflet:January 2020
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NETDEX 3 mg/mL + 1 mg/mL Eye Drops in Single Dose ContainersDosage form: EYEDROP, 1 mg/ml + 5 mg/mlActive substance: dexamethasone and antiinfectivesManufacturer: Santen OyPrescription requiredDosage form: EYEDROP, -Active substance: dexamethasone and antiinfectivesManufacturer: Novartis Farmaceutica S.A.Prescription requiredDosage form: EYEDROP, 3mg/ml + 1mg/mlActive substance: dexamethasone and antiinfectivesManufacturer: Sifi S.P.A.Prescription required
Online doctors for NETDEX 3 mg/mL + 1 mg/mL Eye Drops in Single Dose Containers
Discuss questions about NETDEX 3 mg/mL + 1 mg/mL Eye Drops in Single Dose Containers, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















