NEMLUVIO 30 mg powder and solvent for injectable solution in pre-filled pen

How to use NEMLUVIO 30 mg powder and solvent for injectable solution in pre-filled pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Nemluvio 30 mg powder and solvent for solution for injection in pre-filled pen
nemolizumab
This medicine is subject to additional monitoring, which will allow for quicker identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Nemluvio and what is it used for
- What you need to know before you use Nemluvio
- How to use Nemluvio
- Possible side effects
- Storage of Nemluvio
- Contents of the pack and other information
1. What is Nemluvio and what is it used for
Nemluvio contains the active substance nemolizumab, a monoclonal antibody (a specialized protein that recognizes a specific target and binds to it).
Nemluvio is used in adults and adolescents from 12 years of age to treat moderate to severe atopic dermatitis (also called atopic eczema when the skin is itchy, red, and dry). It can be used when patients can receive systemic treatments (a medicine given by mouth or by injection).
Nemluvio is also used in adults to treat moderate to severe prurigo nodularis (PN), also called chronic prurigo nodularis (CPN), a long-term skin condition associated with a rash that causes itching. It is used when patients can receive systemic treatments.
Nemolizumab, the active substance in Nemluvio, blocks the action of a protein called interleukin (IL)-31. IL-31 plays an important role in the inflammation and itching of the skin seen in people with atopic dermatitis and prurigo nodularis. By blocking IL-31, this medicine can reduce these symptoms.
2. What you need to know before you use Nemluvio
Do not use Nemluvio
- If you are allergic to nemolizumab or any of the other ingredients of this medicine (listed in section 6).
If you think you may be allergic or are not sure, talk to your doctor, pharmacist, or nurse before using Nemluvio.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting to use Nemluvio.
Traceability
It is important to keep a record of the batch number of Nemluvio. Each time you get a new pack of Nemluvio, write down the date and batch number (which is stated on the pack after "Batch") and keep this information in a safe place.
Allergic reactions
Nemluvio may cause allergic reactions (hypersensitivity) which can be serious. Allergic reactions happen soon after using this medicine but can also happen later. You must be alert to the signs of these reactions while you are using Nemluvio. These can be:
- breathing problems
- swelling of the face, mouth, and tongue
- fainting, dizziness, or feeling dizzy due to low blood pressure
- hives
- itching
- skin rash
If you notice any signs of an allergic reaction, stop using Nemluvio and tell your doctor or seek medical attention immediately.
Worsening of asthma
If you have a severe respiratory disease such as asthma, chronic obstructive pulmonary disease (COPD), or chronic bronchitis, talk to your doctor before taking Nemluvio. If your respiratory disease gets worse after starting treatment with Nemluvio, tell your doctor immediately.
Vaccination
It is recommended to complete the recommended vaccination schedule in your case before starting to take Nemluvio. You should avoid vaccination with live vaccines while using Nemluvio. Talk to your doctor about your current vaccination schedule.
Children and adolescents
- Do not give this medicine to children with atopic dermatitis under 12 years of age and with a body weight below 30 kg; it has not been studied in this age group.
- Do not give this medicine to children and adolescents with prurigo nodularis under 18 years of age; it has not been studied in this age group.
Other medicines and Nemluvio
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
- Tell your doctor or pharmacist if you have been vaccinated recently or are going to be vaccinated.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
The effects of this medicine in pregnant women are not known; therefore, it is preferable to avoid using Nemluvio during pregnancy unless your doctor advises you to use it.
Breast-feeding
It is not known whether Nemluvio passes into breast milk. Nemluvio may pass into breast milk in the first few days after giving birth. Therefore, you should tell your doctor if you are breast-feeding or planning to breast-feed, so that you and your doctor can decide if you can use Nemluvio.
Driving and using machines
Nemluvio is unlikely to affect your ability to drive or use machines.
3. How to use Nemluvio
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are not sure, talk to your doctor or pharmacist again.
Treatment should be started and supervised by a doctor who has experience in the diagnosis and treatment of atopic dermatitis and prurigo nodularis.
How much Nemluvio to use and for how long
Your doctor will decide how much Nemluvio you need and for how long you will use it.
Adult patients and adolescents with atopic dermatitis (from 12 years of age)
The recommended dose of Nemluvio is:
- A first dose of 60 mg (two 30 mg injections)
- Subsequent doses of 30 mg every 4 weeks for 16 weeks
After 16 weeks of treatment, your doctor will check the effectiveness of the medicine for you. If the doctor decides that continuing to use it will be beneficial for you, you will continue to take a dose of 30 mg every 8 weeks.
Nemluvio can be used with or without topical eczema medicines.
Adult patients with prurigo nodularis (PN)
The recommended dose is based on body weight.
If you weigh less than 90 kg:
- A first dose of 60 mg (two 30 mg injections)
- Subsequent doses of 30 mg every 4 weeks
If you weigh 90 kg or more:
- A first dose of 60 mg (two 30 mg injections)
- Subsequent doses of 60 mg (two 30 mg injections) every 4 weeks
After 16 weeks of treatment, your doctor will check the effectiveness of the medicine for you to decide if continuing to use it will be beneficial for you.
How to use Nemluvio
Read carefully the instructions for use included at the end of this leaflet before using Nemluvio. The instructions show step-by-step how to use the medicine.
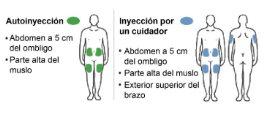
Nemluvio is given as an injection under the skin (subcutaneous injection) using the pre-filled pen. It should be injected into the upper front part of the thigh or into the abdomen, avoiding the area around the navel (5 cm around). If someone else is giving you the injection, it can also be given into the upper arm.
You and your doctor or nurse will decide if you can inject this medicine yourself. You will only be able to inject yourself after your doctor or nurse has taught you how to do it. A caregiver can also give you the injection after they have received proper training.
It is recommended to change the injection site with each injection. Nemluvio should not be injected into areas of sensitive, inflamed, swollen, tender, or damaged skin, or skin with bruises, scars, or open wounds.
If you use more Nemluvio than you should
If you have used more Nemluvio than you should or have taken the next dose too soon, talk to your doctor, pharmacist, or nurse.
If you forget to use Nemluvio
Do not take a double dose to make up for a forgotten dose. If you forget to inject a dose of Nemluvio, inject it as soon as possible and continue with your original schedule.
If you stop using Nemluvio
Do not stop using Nemluvio without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Common(may affect up to 1 in 10 people)
Nemluvio may cause allergic reactions (hypersensitivity). Stop using Nemluvio and tell your doctor or seek medical attention immediately if you notice any signs of an allergic reaction.The signs can be, among others:
- breathing problems
- swelling of the face, mouth, and tongue
- fainting, dizziness, or feeling dizzy due to low blood pressure
- hives
- itching
- skin rash
Other side effects
Common(may affect up to 1 in 10 people)
- Fungal skin infections such as corporal dermatophytosis (body ringworm) or athlete's foot, fungal nail infections, and jock itch
- Headache
- Worsening of asthma (in people with pre-existing asthma)
- Eczema
- Atopic dermatitis (skin with itching, redness, and dryness in people prone to allergies)
- Nummular eczema (a skin condition that causes round or oval patches of inflamed skin with itching and dryness)
- Injection site reactions, including redness, itching, bruising, pain, irritation, and swelling at the injection site
Uncommon(may affect up to 1 in 100 people)
- Increased number of white blood cells, which can be seen in a blood test (eosinophilia)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nemluvio
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Keep the pre-filled pen in the original packaging to protect it from light.
If necessary, Nemluvio can be stored at room temperature (up to 25°C) for a single period of 90 days at most. Write down the date you took the pen out of the refrigerator in the space provided on the outer carton. Do not use Nemluvio if it is past the expiry date or if 90 days have passed since it was taken out of the refrigerator (whichever comes first).
Once the steps for reconstitution are completed, Nemluvio must be used within 4 hours or discarded.
Do not use this medicine if you notice that the powder is not white.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Nemluvio
- The active substance is nemolizumab. Each single-use pre-filled pen contains 30 mg of nemolizumab.
- The other components are:
- Powder:sucrose, trometamol, trometamol hydrochloride (for pH adjustment), arginine hydrochloride, poloxamer 188.
- Solvent:water for injections.
Appearance of the Product and Container Contents
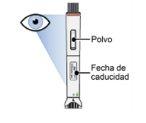
Nemluvio powder and solvent for solution for injection in a pre-filled pen consists of a single-use pre-filled pen containing a glass cartridge that supplies a white powder and a clear, colorless liquid. The liquid is not visible from the inspection window before dissolution.
Nemluvio is presented in a 30 mg pre-filled pen in a pack containing 1 pre-filled pen or in multiple packs containing 2 or 3 boxes, each with 1 pre-filled pen.
Only certain pack sizes may be marketed.
Marketing Authorization Holder
Galderma International
La Defense 4, Tour Europlaza
20 Avenue Andre Prothin
92927 Paris La Defense Cedex
France
Manufacturers
Q-Med AB
Seminariegatan 21
Uppsala Lan
752 28 Uppsala
Sweden
Nuvisan France S.A.R.L.
2400 Route Des Colles
06410 Biot
France
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Luxembourg/Luxemburg Galderma Benelux BV Tél/Tel: +31 183691919 e-mail: [email protected] | Ireland Galderma (UK) Ltd. Tel: +44 (0)300 3035674 e-mail: [email protected] |
| Latvija
Tel: +371/67/103205 e-mail: [email protected] |
Ceská republika Slovenská republika Galenoderm s.r.o. Tel: +421 2 49 10 90 10 e-mail: [email protected] | Lietuva
Tel: +370/52/711710 e-mail: [email protected] |
Danmark Norge Ísland Suomi/Finland Sverige Galderma Nordic AB Tlf/Sími/Puh/Tel: + 46 18 444 0330 e-mail: [email protected] | Magyarország Ewopharma Hungary Kft. Tel.: +36 1 200 4650 e-mail: [email protected] |
Deutschland Galderma Laboratorium GmbH Tel: + 49 (0) 800 – 5888850 e-mail: [email protected] | Malta Prohealth Limited Tel. +356 21461851, +356 21460164 e-mail: [email protected] |
Eesti
Tel: + 372/6/460980 e-mail: [email protected] | Nederland Galderma Benelux BV Tel: + 31 183691919 e-mail: [email protected] |
Ελλáδα Κúπρος Pharmassist Ltd Τηλ: + 30 210 6560700 e-mail: [email protected] | Österreich Galderma Austria GmbH Tel: 0043 732 715 993 e-mail: [email protected] |
España Laboratorios Galderma SA Tel: + 34 902 02 75 95 e-mail: [email protected] | Polska Galderma Polska Sp. z o.o. Tel.: + 48 22 331 21 80 e-mail: [email protected] |
France Galderma International Tél: +33 (0)1 58 86 45 45 e-mail: [email protected] | Portugal Laboratorios Galderma SA – Sucursal em Portugal Tel: + 351 21 315 19 40 e-mail: [email protected] |
Hrvatska Medical Intertrade d.o.o. T: +385 1 333 6036 e-mail: [email protected] | România Neola pharma SRL Tel: + 40 21 233 17 81 e-mail: [email protected] |
Italia Galderma Italia S.p.A. Tel: +39 3371176197 e-mail: [email protected] | Slovenija Medical Intertrade d.o.o. T: +386 1 2529 113 F: +386 1 2529 114 e-mail: [email protected] |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
-----------------------------------------------------------------------------------------------------------------------------------
Instructions for Use
IMPORTANT: Read the leaflet before using this medicinal product. This pen requires specific steps before injection.
Nemluvio 30 mg powder and solvent for solution for injection in a pre-filled pen (nemolizumab)
Do not inject Nemluvio or have it injected by another person until a healthcare professional has taught you how to do it.
In case of doubt, consult a healthcare professional.
Nemluvio is presented in a single-use, dual-chamber pre-filled pen (referred to as "Nemluvio pen" or "pen" in these instructions).
The pen contains two chambers, one with the medicinal product (the powder) and the other with water for dissolving the powder.
Before injecting the medicinal product, you must mix the powder with the water by following the description below.
General Description of the Device
Nemluvio pre-filled pen, dual-chamber
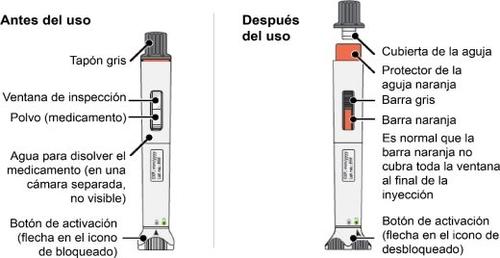
Important Information
What you need to know before use
- Read all the instructions carefully before using the Nemluvio pen.
- Mark your calendarin advance to remember when you need to receive Nemluvio.
- Follow all the steps exactly as described. This will ensure you receive the correct dose of the medicinal product.
- Do notuse the Nemluvio pen if it has been dropped onto a hard surface or if it is damaged, cracked, or broken.
Storage Information
- Keep the Nemluvio pen and all medicinal products out of the sight and reach of children.
- Store the Nemluvio pen in the refrigerator at 2 °C to 8 °C.
- Do notfreeze the Nemluvio pen.
- Keep the Nemluvio pen in the original packaging to protect it from light.
- The Nemluvio pen can be stored in the original packaging at room temperature up to 25 °C for a single period of 90 days at most. If you take it out of the refrigerator, note the date of removal on the carton and use Nemluvio within 90 days.
- Do not use Nemluvio if it has passed the expiry date or if 90 days have passed since it was taken out of the refrigerator (whichever occurs first).
- Once the reconstitution steps are completed, Nemluvio must be used within 4 hours.
Step 1: Allow Nemluvio to reach room temperature Cold medicinal product injections can cause pain at the injection site. Remove the Nemluvio carton from the refrigerator and wait for 30 to 45 minutes for it to reach room temperature before starting with Step 2. Do not:
Note:In some cases, your doctor may prescribe two pens for you to use at the same time. In that case, you must remove two pens and use one pen after the other. | |
Step 2: Wash your hands with soap and dry them well. | |
Step 3: Prepare the supplies Remove the pen from the carton and place the following materials on a clean, flat, and well-lit surface:
Materials not included in the carton. |
|
Step 4: Check the Nemluvio pen to ensure that: | |
Do notuse the pen unless all the above conditions are met. If anyof the conditions are not met, discard the pen and use a new one (see Step 13.5 "Dispose"). |
|
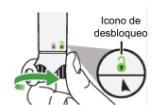
Step 5: Activate the Nemluvio pen Hold the pen in a vertical position and turn the activation button to the right until it stops. This initiates the process of transferring water to the chamber containing the powder. |
|
Step 6: Wait until the gray bar stops moving Observe the inspection window until the gray bar has stopped. Do notshake the pen before the gray bar has stopped completely to allow for the administration of the exact dose. |
|
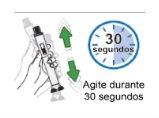
Step 7: Shake to dissolve the medicinal product Once the gray bar has stopped completely, shake the pen up and down for 30 seconds. |
|
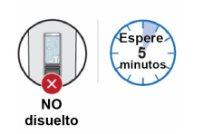
Step 8: Wait 5 minutes for the bubbles to decrease Wait for the bubbles to decrease and for the powder to dissolve completely. This will take about 5 minutes. Note:If the medicinal product has not dissolved completely, shake it again for 30 seconds and wait 5 minutes. Note:It is normal for a small layer of foam or a few small air bubbles to remain in the dissolved medicinal product. |
|
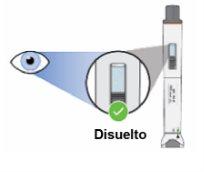
Step 9: Check the medicinal product in the inspection window Check that the dissolved medicinal product:
Do notuse the pen if the dissolved medicinal product is cloudy or contains particles. Discard the pen and use a new one (see Step 13.5 "Dispose"). Note:Once the medicinal product is dissolved, it must be used within 4 hours. During this time, it must be kept at room temperature (up to 25 °C). If it is not used within 4 hours, discard it. |
|
| |
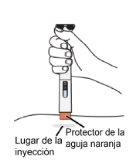
Step 10: Choose an injection site The injection can be administered in the abdomen or in the upper thigh. A caregiver can also administer the injection in the upper outer arm. Do notinject:
orin areas of sensitive skin, with bruises, redness, or scars or stretch marks.
|
|
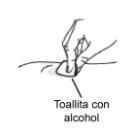
Step 11: Clean the injection site
Do not:
orfan or blow on the cleaned injection site.
|
|
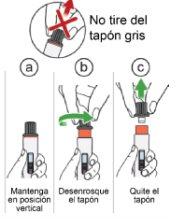
Step 12: Turn the gray cap to expose the needle protector
Unscrew the gray cap until the orange needle protector appears.
Once the cap is removed, discard it in a sharps disposal container (see Step 13.5 "Dispose"). Do not:
ortouch the orange needle protector. Note:If you cannot remove the cap, go back to Step 5and ensure the activation button is fully turned to the right until it stops. |
|
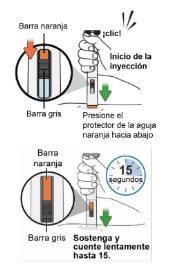
Step 13: Inject the medicinal product
Note:Make sure you can easily see the inspection window during the injection.
The injection starts immediately with a click. You should see the orange and gray bars moving. Continuepushing the pen down for 15 seconds.
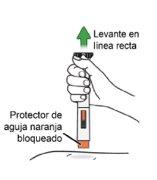
This means the injection is complete. Do notlift the pen until the orange and gray bars have stopped moving. If the orange bar is not visible, discard the pen and use a new one (see Step 13.5 "Dispose"). Note:It is normal for the orange bar not to cover the entire inspection window at the end of the injection.
The orange needle protector will lock to cover the needle. Note:If bleeding occurs, apply pressure to the injection site with a cotton ball or swab. Do notrub the injection site.
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEMLUVIO 30 mg powder and solvent for injectable solution in pre-filled penDosage form: INJECTABLE, 1 mlActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 300 mgActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: CAPSULE, 10 mgActive substance: alitretinoinManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for NEMLUVIO 30 mg powder and solvent for injectable solution in pre-filled pen
Discuss questions about NEMLUVIO 30 mg powder and solvent for injectable solution in pre-filled pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions