
NEBIVOLOL SANDOZ 5 mg TABLETS

How to use NEBIVOLOL SANDOZ 5 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Nebivolol Sandoz 5 mg Tablets EFG
Nebivolol
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Nebivolol Sandoz and what is it used for
- What you need to know before taking Nebivolol Sandoz
- How to take Nebivolol Sandoz
- Possible side effects
- Storage of Nebivolol Sandoz
- Contents of the pack and further information
1. What is Nebivolol Sandoz and what is it used for
Nebivolol Sandoz contains nebivolol, a cardiovascular medication that belongs to the group of selective beta blockers (i.e., with a selective action on the cardiovascular system). It prevents an increase in heartbeats and controls the heart's pumping force. It also has a dilating effect on blood vessels, which contributes to lowering blood pressure.
It is used in the treatment of high blood pressure (hypertension).
Nebivolol is also used to treat mild to moderate chronic heart failure in patients aged 70 or older, in combination with other therapies.
2. What you need to know before taking Nebivolol Sandoz
Do not takeNebivolol Sandoz
- If you are allergic (hypersensitive) to nebivolol or any of the other ingredients of this medication (listed in section 6).
- If you have one or more of the following conditions:
- low blood pressure,
- severe circulation problems in arms or legs,
- very slow heart rate (less than 60 heartbeats per minute),
- other severe heart rhythm problems (e.g., second- and third-degree atrioventricular block, heart conduction disorders),
- heart failure that has recently occurred or worsened, or you are receiving intravenous treatment for circulatory shock due to acute heart failure to help your heart function,
- asthma or difficulty breathing (currently or in the past),
- untreated pheochromocytoma, a tumor located in the kidneys (in the adrenal glands),
- liver function disorders,
- a metabolic disorder (metabolic acidosis), such as diabetic ketoacidosis.
Warnings and precautions
Tell your doctor if you have or develop any of the following problems:
- abnormally slow heart rate,
- a type of chest pain due to the spontaneous occurrence of cardiac spasms called Prinzmetal's angina,
- untreated chronic heart failure,
- first-degree heart block (a type of mild conduction disorder that affects heart rhythm),
- poor circulation in arms or legs, e.g., Raynaud's disease or syndrome, cramp-like pains when walking,
- prolonged respiratory problems,
- diabetes: this medication has no effect on blood sugar, but it can mask the warning signs of low blood sugar (e.g., palpitations, rapid heartbeat) and may increase the risk of severe hypoglycemia when used with certain oral antidiabetic medications called sulfonylureas (e.g., gliquidone, gliclazide, glibenclamide, glipizide, glimepiride, or tolbutamide),
- overactive thyroid gland: this medication can mask the signs of an abnormally rapid heartbeat due to this disorder,
- allergies: this medication can intensify your reaction to pollen or other substances you are allergic to,
- psoriasis (a skin disease - scaly pink patches) or if you have ever had psoriasis,
If you have severe kidney problems, do not take nebivolol for heart failure, and inform your doctor.
At the start of your treatment for chronic heart failure, you will be regularly monitored by an experienced doctor (see section 3).
This treatment should not be stopped suddenly unless it is clearly indicated by your doctor (see section 3).
Children and adolescents
Nebivolol is notrecommended for use in children and adolescents due to a lack of data.
Taking Nebivolol Sandoz with other medications
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication, includingthose obtained without a prescription. Certain medications cannot be used at the same time, while others require specific changes (e.g., in dosage).
Always inform your doctor if, in addition to nebivolol, you are using any of the following medications:
- Medications for blood pressure control or heart problems (such as amiodarone, amlodipine, cibenzoline, clonidine, digoxin, diltiazem, disopyramide, felodipine, flecainide, guanfacine, hydroquinidine, lacidipine, lidocaine, methyldopa, mexiletine, moxonidine, nicardipine, nifedipine, nimodipine, nitrendipine, propafenone, quinidine, rilmenidine, verapamil).
- Sympathomimetic agents (medications that mimic the effects of sympathetic activation of the heart and circulation).
- Sedatives and therapies for psychosis (a mental illness), e.g., barbiturics (also used for epilepsy), phenothiazine (also used for vomiting and nausea), and thioridazine.
- Medications for diabetes, such as insulin or oral antidiabetics.
- Medications used for depression, e.g., amitriptyline, paroxetine, fluoxetine.
- Medications used for anesthesia during an operation.
- Medications for asthma, nasal decongestants, and some medications for treating eye disorders such as glaucoma (increased eye pressure) or pupil dilation.
- Amifostine used during cancer treatment.
- Baclofen used to treat epilepsy.
All these medications, as well as nebivolol, can influence blood pressure and/or heart function.
- Medications to treat excess acidity or stomach ulcers (antacids), e.g., cimetidine; you should take nebivolol during meals and the antacid between meals.
Taking Nebivolol Sandoz with food and drinks
Nebivolol can be taken with meals or on an empty stomach, but it is best to take the tablet with a little water.
Pregnancy and breastfeeding
Nebivolol should not be taken during pregnancy, unless clearly necessary.
Its use is not recommended during breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using a medication.
Driving and using machines
This medication can cause dizziness or fatigue. If you notice it affects you, do notdrive or use machines.
Nebivolol Sandoz contains lactose and sodium
This medication contains lactose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
This medication contains less than 23 mg of sodium (1 mmol) per tablet; this is essentially "sodium-free".
3. How to take Nebivolol Sandoz
Follow the instructions for this medication of nebivolol as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Nebivolol can be taken before, during, or after meals, as well as outside of meals. It is best to take the tablet with a little water.
Treatment of high blood pressure (hypertension)
- The recommended dose is 1 tablet per day. The dose should be taken preferably at the same time each day.
- Elderly patients and patients with kidney disorders will normally start treatment with ½ (half) tablet per day.
- The therapeutic effect on blood pressure is evident after 1-2 weeks of treatment. In some cases, the optimal effect is only achieved after 4 weeks of treatment.
Treatment of chronic heart failure
- Treatment must be initiated and strictly controlled by an experienced doctor.
- Your doctor will have you start treatment with ¼ (a quarter) of a tablet per day. This dose may be increased after 1-2 weeks to ½ (half) tablet per day, then to 1 tablet per day, and finally to 2 tablets per day to reach the suitable dose for you. Your doctor will prescribe the correct dose at each step, and you must follow their instructions exactly.
- The maximum recommended dose is 2 tablets (10 mg) per day.
- The start of treatment and each dose increase will be done under the supervision of an experienced doctor for a period of at least 2 hours.
- If necessary, your doctor may reduce the dose.
- Do not stop treatment abruptly, as this could worsen heart failure.
- Patients with severe kidney problems should not take this medication.
- Take your medication once a day, preferably at the same time each day.
Instructions for dividing the tablets
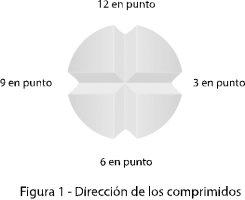
If your doctor has indicated that you should take ¼ or ½ (half) of a tablet, place the tablet on a flat, hard surface, such as a table, with the face with the score lines facing up.
- Place the tablet on a flat, hard surface, such as a table, so that the face with the four-leaf clover shape is facing up and the score lines of the tablet coincide with 12 o'clock, 3 o'clock, 6 o'clock, and 9 o'clock (Figure 1).
- Place your thumb on the surface of the tablet so that your thumb is from 3 o'clock to 9 o'clock (Figure 2).
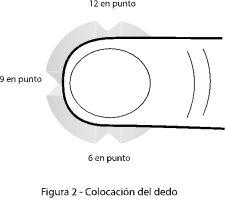
- Apply uniform pressure to the surface of the tablet until it breaks.
Your doctor may decide to combine nebivolol tablets with other medications to treat your condition.
Not to be used in children or adolescents.
If you take more Nebivolol Sandoz than you should
If you have accidentally taken an overdose of this medication, consult your doctor or pharmacist immediately. The most frequent symptoms and signs of a nebivolol overdose are very slow heart rate (bradycardia), low blood pressure with possible fainting (hypotension), wheezing breathing like asthma (bronchospasm), and acute heart failure.
You can take activated charcoal (available at your pharmacy) while waiting for your doctor to arrive.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicological Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take Nebivolol Sandoz
If you have forgotten to take a dose of nebivolol, but you remember a little later when you should have taken it, take it as usual. However, if a long time has passed (e.g., several hours), so that the next dose is near, skip the missed dose and take the next normal doseat the usual time. Do not take a double dose. You should try to avoid repeatedly forgetting to take your medication.
If you stop treatment with Nebivolol Sandoz
You should always consult your doctor before stopping treatment with nebivolol when you are taking it for high blood pressure or chronic heart failure.
Do not stop treatment with nebivolol abruptly, as this could temporarily worsen your heart failure. If it is necessary to stop treatment with nebivolol for chronic heart failure, the daily dose should be gradually reduced, based on reducing the dose by half at weekly intervals.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
When nebivolol is used to treat high blood pressure, the possible side effects are:
Common side effects (may affect up to 1 in 10 people):
- headache,
- dizziness,
- fatigue,
- strange itching or tingling sensation,
- diarrhea,
- constipation,
- nausea,
- wheezing breathing,
- swelling of hands or feet.
Uncommon side effects (may affect up to 1 in 100 people):
- decreased heart rate or other heart disorders,
- low blood pressure,
- cramp-like pains when walking,
- altered vision,
- impotence,
- feeling of depression,
- difficult digestion (dyspepsia), stomach or intestinal gas, vomiting,
- skin rashes, itching,
- wheezing breathing like asthma due to sudden spasms in the muscles around the respiratory tract (bronchospasm),
- nightmares.
Rare side effects (may affect less than 1 in 10,000 people):
- fainting,
- worsening of psoriasis (a skin disease, scaly pink patches).
Frequency not known (cannot be estimated from available data)
- Hypersensitivity,
- angioneurotic edema (swelling of the face, lips, mouth, tongue, or throat),
- urticaria (skin rash).
The following side effects have also been reported with similar medications:
- hallucinations,
- psychosis,
- confusion,
- cold or cyanotic (blue or purple) extremities,
- Raynaud's phenomenon (discoloration of fingers, toes, and occasionally other areas),
- dry eyes,
- formation of new connective tissue in the eyes and diaphragm (practolol-like mucocutaneous toxicity).
In a clinical study for chronic heart failure, the observed side effectswere:
Very common side effects (may affect more than 1 in 10 people):
- low heart rate,
- dizziness.
Common side effects (may affect up to 1 in 10 people):
- worsening of heart failure,
- low blood pressure (like a feeling of fainting when standing up quickly),
- inability to tolerate this medication,
- a type of mild heart conduction disorder that affects heart rhythm (first-degree AV block),
- swelling of the legs (swelling of the ankles).
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
.
5. Storage of Nebivolol Sandoz
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date stated on the packaging after CAD/EXP. The expiration date is the last day of the month indicated.
This medication does not require special storage conditions.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and any unused medication in the SIGRE collection point at your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Composition ofNebivolol Sandoz
- The active ingredient is nebivolol. Each tablet contains 5 mg of nebivolol (as hydrochloride).
- The other components are: sodium croscarmellose, lactose monohydrate, cornstarch, microcrystalline cellulose, hypromellose 5 cps, anhydrous colloidal silica, magnesium stearate.
Appearance of the Product and Package Contents
White or almost white tablets with a four-leaf clover shape on one side, convex on the other side, with a quadrangular shape on both sides, with break marks on both sides (diameter: 9 mm).
The tablets are packaged in PVC/aluminum blisters and polyethylene bottles with a tamper-evident seal, packaged in a cardboard box.
Package Sizes
Blister: 7, 10, 14, 20, 28, 30, 50, 56, 60, 84, 90, 98, 100, and 500 tablets.
Bottle: 7, 10, 14, 20, 28, 30, 50, 56, 60, 84, 90, 98, 100, and 500 tablets.
Not all formats may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Sandoz Farmacéutica, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
Spain
Manufacturer:
LEK S.A.
Ul. Domaniewska 50 C, 02-672 Warszawa
Poland
or
Lek Pharmaceuticals d.d.
Verovskova 57, 1526 Ljubljana
Slovenia
or
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1, 39179 Barleben
Germany
or
Lek S.A.
16 Podlipie Str.
95-010 Stryków
Poland
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: Nebivolol Sandoz 5 mg Tabletten
Belgium: Nebivolol Sandoz 5 mg tabletten
Bulgaria: Nebivolol Sandoz 5 mg tablets
Czech Republic: Nebivolol Sandoz 5 mg tablety
France: NEBIVOLOL Sandoz 5 mg, comprimé quadrisécable
Italy: NEBIVOLOLO SANDOZ 5 mg compresse
Netherlands: Nebivolol Sandoz 5 mg tabletten
Poland: NebivoLEK 5 MG, TABLETKI
Portugal: Nebivolol Sandoz 5 mg comprimido
United Kingdom: Nebivolol 5mg Tablets
Date of the last revision of this leaflet:April 2024
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.87 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEBIVOLOL SANDOZ 5 mg TABLETSDosage form: TABLET, 10 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: TABLET, 2.5 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: TABLET, 5 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription required
Online doctors for NEBIVOLOL SANDOZ 5 mg TABLETS
Discuss questions about NEBIVOLOL SANDOZ 5 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











