
INSUCOR 2.5 mg TABLETS

How to use INSUCOR 2.5 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Insucor 2.5 mg Tablets
nebivolol
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Insucor 2.5 mg Tablets are and what they are used for
- What you need to know before you take Insucor 2.5 mg Tablets
- How to take Insucor 2.5 mg Tablets
- Possible side effects
- Storage of Insucor 2.5 mg Tablets
- Contents of the pack and further information
1. What Insucor is and what it is used for
Insucor contains nebivolol, a cardiovascular medicine, belonging to the group of selective beta-blockers (with selective activity in the cardiovascular system). It prevents an increase in heart rate and controls the heart's pumping force. It also has a vasodilating effect on blood vessels, which in turn helps to lower blood pressure.
It is used to treat high blood pressure (hypertension).
Insucor is also used to treat mild and moderate chronic heart failure in patients aged 70 or over, in combination with other medicines.
2. What you need to know before taking Insucor
Do not takeInsucor
- if you are allergic to nebivolol or any of the other ingredients of this medicine (listed in section 6).
- if you have any of the following conditions:
- low blood pressure
- severe circulation problems in your arms or legs.
- very slow heart rate (less than 60 beats per minute)
- other severe heart rhythm disorders (e.g. second and third degree atrioventricular block, cardiac conduction disorders).
- heart failure that has recently worsened or if you are receiving intravenous treatment to help your heart work after suffering a circulatory collapse due to acute heart failure.
- asthma or difficulty breathing (currently or in the past), pheochromocytoma, a tumor located in the upper part of the kidneys (adrenal glands) that is not being treated.
- liver function disorder.
- metabolic disorder (metabolic acidosis), such as diabetic ketoacidosis.
Warnings and precautions
Consult your doctor or pharmacist before starting to take Insucor.
Tell your doctor if you have or develop any of the following problems:
- abnormally slow heart rate
- a type of chest pain due to a spontaneous spasm of the heart arteries, called Prinzmetal's angina
- untreated chronic heart failure
- first-degree heart block (a type of mild cardiac conduction disorder that affects the heart rhythm)
- poor circulation in arms or legs, such as Raynaud's disease or pain when walking similar to a cramp.
- chronic respiratory problems.
- diabetes: this medicine has no effect on blood sugar levels, but it can mask the warning signs produced by a decrease in these levels (e.g. palpitations, rapid heart rate) and may increase the risk of severe hypoglycemia when used with certain antidiabetic medicines called sulfonylureas (e.g. gliquidone, gliclazide, glibenclamide, glipizide, glimepiride or tolbutamide).
- overactivity of the thyroid gland: this medicine can mask the symptoms of rapid heart rate due to this condition.
- allergy, this medicine can increase your reaction to pollen or other substances you are allergic to.
If you have severe kidney problems, consult your doctor before taking Insucor to treat your heart failure.
When starting treatment for chronic heart failure, you will be closely monitored by your doctor (see section 3).
This treatment should not be stopped abruptly, unless clearly indicated and evaluated by your doctor (see section 3).
Children and adolescents
Do notuse Insucor in children and adolescents due to the lack of data on the use of this medicine in these patients.
Other medicines and Insucor
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Always tell your doctor if, in addition to Insucor, you are using any of the following medicines:
- Medicines to control blood pressure or medicines used to treat heart diseases (such as amiodarone, amlodipine, cibenzoline, clonidine, digoxin, diltiazem, disopyramide, felodipine, flecainide, guanfacine, hydroquinidine, lacidipine, lidocaine, methyldopa, mexiletine, moxonidine, nicardipine, nifedipine, nimodipine, nitrendipine, propafenone, quinidine, rilmenidine, verapamil)
- Sedatives and therapies used in the treatment of psychosis (mental illness) e.g. barbiturics (also used to treat epilepsy), phenothiazine (also used for vomiting and nausea) and thioridazine.
- Medicines used to treat depression e.g. amitriptyline, paroxetine and fluoxetine.
- Medicines used for anesthesia during an operation.
- Medicines used to treat asthma, nasal congestion or certain eye disorders such as glaucoma (increased pressure in the eye) or pupil dilation.
- Baclofen (an antispastic medicine); amifostine (a protective medicine used during cancer treatment).
- Medicines for diabetes, such as insulin or oral antidiabetics.
All these medicines, like nebivolol, can affect blood pressure and/or heart function.
- Antimalarials (mefloquine).
- Medicines to treat excess stomach acid or ulcers (antacids): you should take Insucor during meals, and the antacid between meals.
Taking Insucor with food and drinks
See section 3.
Pregnancy and breast-feeding
Pregnancy
Insucor should not be taken during pregnancy unless clearly necessary.
Breast-feeding
It is not recommended during breast-feeding.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
This medicine may cause dizziness or fatigue. If this happens, do notdrive or use machines.
Insucor contains lactose
This medicine contains
Insucor contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per film-coated tablet; this is, essentially “sodium-free”.
3. How to take Insucor
Follow exactly the administration instructions of this medicine given by your doctor. In case of doubt, consult your doctor or pharmacist again.
Insucor can be taken before, during or after meals, but it can also be taken independently of them. It is preferable to take the tablet with a little water.
Treatment of high blood pressure (hypertension)
- The usual dose is 5 mg (two tablets) per day. It is preferable to take the dose always at the same time of day.
- In elderly patients and/or with kidney problems, it is recommended to start treatment with a 2.5 mg tablet per day.
- The therapeutic effect on blood pressure is achieved after 1 to 2 weeks of treatment. Occasionally, the optimal effect is only achieved after 4 weeks.
- If you are over 75 years old, you may need closer monitoring by your doctor.
Treatment of chronic heart failure
The treatment will be closely controlled by your doctor.
- Your doctor will start treatment with 1.25 mg (half a tablet) per day. This dose may be increased after one to two weeks to 2.5 mg (one 2.5 mg tablet) per day; then, to 5 mg (two tablets) per day and, subsequently, to 10 mg (four tablets) per day, until the correct dose for you is reached. Your doctor will prescribe the dose that is right for you at each step and you should carefully follow your doctor's instructions.
- The maximum recommended dose is 10 mg.
- When starting treatment and each time the dose is increased, it will be necessary for an experienced doctor to closely monitor you for two hours.
- Your doctor may decrease the dose if necessary.
- You should not stop taking the medicine abruptly, as this could lead to a worsening of heart failure.
- Patients with severe kidney problems should not take this medicine.
- Take your medicine once a day, preferably at the same time of day.
Your doctor may decide that it is necessary to combine these tablets with other medicines to treat your disease.
If your doctor has told you to take ½ (half) a tablet daily, see the instructions below on how to break Insucor 2.5 mg and Insucor 5 mg tablets into equal doses.
- Place the tablets on a flat, hard surface (e.g. a table or countertop), with the break line facing up.
- Break the tablet by pushing it with the index fingers of both hands along the break line (Diagrams 1 and 2).
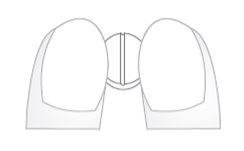
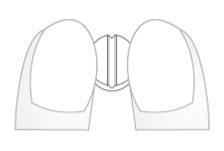 Diagram 1 Diagram 2
Diagram 1 Diagram 2
Diagrams 1 and 2: easy breaking of Insucor 2.5 mg and Insucor 5 mg tablets into equal doses.
Use in children and adolescents
Do not use in children or adolescents.
If you take more Insucor than you should
If you have accidentally taken an overdose of this medicine, consult your doctor or pharmacist immediatelyor call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount taken. The symptoms and signs most frequently reported in an overdose are related to very slow heart rate (bradycardia), low blood pressure with possible fainting (hypotension), difficulty breathing such as in asthma (bronchospasm) and acute heart failure.
You can take activated charcoal (available at your pharmacy) while waiting for your doctor to arrive.
If you forget to take Insucor
If you forget to take a dose of Insucor, but remember soon after when you should have taken it, take the daily dose as usual. If a lot of time has passed (several hours), so that it is close to the next dose, skip the missed dose and take the next scheduled doseat the usual time. Do not take a double dose. However, you should try to avoid repeatedly forgetting to take the medicine.
If you stop taking Insucor
Always consult your doctor before stopping treatment with Insucor, whether you are taking it for high blood pressure or chronic heart failure.
Do not stop treatment abruptly, as this could temporarily worsen your heart failure.
If it is necessary to stop treatment for chronic heart failure, the daily dose should be gradually decreased, starting by halving the dose at weekly intervals. If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking nebivolol and talk to your doctor immediately if you have any of the following side effects:
- allergic reactions throughout the body, with generalized skin rashes (hypersensitivity reactions)
- sudden swelling, especially around the lips, eyelids and/or tongue, which may be accompanied by acute difficulty breathing (angioedema).
- Skin rash characterized by pink, raised patches that itch, of allergic or non-allergic cause (urticaria)
- Difficulty breathing like in asthma, due to a sudden contraction of the muscles surrounding the airways. Feeling of tightness in the chest, difficulty breathing or wheezing (bronchospasm)
When Insucor is used to treat high blood pressure, its possible side effects are:
Common side effects (may affect up to 1 in 10 people):
- headache.
- dizziness.
- fatigue.
- unusual sensation of itching or tingling.
- diarrhea.
- constipation.
- nausea.
- shortness of breath.
- swelling of the hands or feet.
Uncommon side effects (may affect up to 1 in 100 people):
- slow heart rate or other heart disorders.
- low blood pressure.
- pain when walking similar to a cramp.
- abnormal vision.
- impotence (difficulty achieving an erection).
- feelings of depression.
- digestive difficulties (dyspepsia), stomach or intestinal gas (indigestion).
- vomiting.
- skin rash, itching.
- difficulty breathing like in asthma, due to a sudden contraction of the muscles surrounding the airways. Feeling of tightness in the chest, difficulty breathing or wheezing (bronchospasm).
- nightmares.
Rare side effects (may affect up to 1 in 10,000 people):
- fainting.
- worsening of psoriasis (a skin disease characterized by scaly pink patches).
The following side effects have been reported in isolated cases during treatment with this medicine:
- allergic reactions throughout the body, with generalized skin rashes (hypersensitivity reactions)
- sudden swelling, especially around the lips, eyelids and/or tongue, which may be accompanied by acute difficulty breathing (angioedema).
- Skin rash characterized by pink, raised patches that itch, of allergic or non-allergic cause (urticaria)
In a clinical study for chronic heart failure, the following side effects were observed:
Very common side effects (may affect more than 1 in 10 people):
- slow heart rate.
- dizziness.
Common side effects (may affect up to 1 in 10 people):
- worsening of heart failure.
- low blood pressure (such as feeling dizzy when standing up quickly).
- intolerance to this medicine.
- mild cardiac conduction disorder that affects the heart rhythm (first-degree atrioventricular block).
- swelling of the lower limbs (such as swelling of the ankles).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Insucor
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP.
The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Return any unused medicine to a pharmacy for disposal. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
If the medicine changes color or shows any signs of deterioration, ask your pharmacist for advice on what to do.
6. Packaging Content and Additional Information
Composition of Insucor
The active ingredient is nebivolol.
Each tablet contains 2.5 mg of nebivolol, equivalent to 2.725 mg of nebivolol hydrochloride.
The other components are:
Lactose monohydrate, corn starch, sodium croscarmellose, hypromellose, microcrystalline cellulose, anhydrous colloidal silica, and magnesium stearate.
Appearance of the Product and Packaging Content
Biconvex, uncoated tablets with a capsule shape and white color, with a score line on one side and smooth on the other.
The tablet can be divided into equal doses.
Insucor tablets are presented in blisters containing 14, 28, 30, 50, 90, 98, 100 tablets.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing authorization holder:
Glenmark Arzneimittel GmbH
Industriestr. 31
82194 Gröbenzell
Germany
Manufacturer:
Glenmark Pharmaceuticals s.r.o.
Fibichova 143
56617 Vysoke Myto
Czech Republic
Glenmark Generics (Europe) Ltd.
The Old Sawmill, Hatfield Park
Hatfield, Herts., AL9 5PG
United Kingdom
Further information about this medicinal product can be obtained from the local representative of the marketing authorization holder:
Glenmark Farmacéutica, S.L.U.
C/ Retama 7, 7ª planta
28045 Madrid
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany Nebivolol Glenmark 2.5 mg tablets
Hungary Nebitrix 2.5 mg
Netherlands Nebivolol Glenmark 2.5 mg tablets
Slovakia Nebitrix 2.5 mg
Spain INSUCOR 2.5 mg tablets
Date of the last revision of this leaflet: April 2025
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es//
- Country of registration
- Average pharmacy price3.93 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to INSUCOR 2.5 mg TABLETSDosage form: TABLET, 10 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: TABLET, 5 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: TABLET, 5 mgActive substance: nebivololManufacturer: Menarini International Operations Luxembourg S.A.Prescription required
Online doctors for INSUCOR 2.5 mg TABLETS
Discuss questions about INSUCOR 2.5 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











