MYRELEZ 120 mg Injectable Solution in Pre-filled Syringe

How to use MYRELEZ 120 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Myrelez 60mg solution for injection in pre-filled syringe EFG
Myrelez 90mg solution for injection in pre-filled syringe EFG
Myrelez 120mg solution for injection in pre-filled syringe EFG
lanreotide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Myrelez and what is it used for
- What you need to know before you use Myrelez
- How to use Myrelez
- Possible side effects
- Storage of Myrelez
- Contents of the pack and other information
1. What is Myrelez and what is it used for
Myrelez contains the active substance lanreotide, which belongs to a group of medicines called growth hormone inhibitors. It is similar to another substance (a hormone) called somatostatin.
Lanreotide decreases hormone levels in the body, such as growth hormone (GH) and insulin-like growth factor-1 (IGF-1), and inhibits the release of some hormones in the gastrointestinal tract and intestinal secretions. Additionally, it has an effect on a type of tumor (called neuroendocrine tumors) of the intestine and pancreas, advanced, by stopping or delaying their growth.
What Myrelez is used for:
- Treatment of acromegaly (a condition in which the body produces too much growth hormone).
- Relief of symptoms, such as hot flashes and diarrhea, that sometimes occur in patients with neuroendocrine tumors (NETs).
- Treatment and control of the growth of some tumors of the intestine and pancreas, called gastroenteropancreatic neuroendocrine tumors or GEP-NETs. It is used when these tumors cannot be removed by surgery.
2. What you need to know before you use Myrelez
Do not use Myrelez:
- if you are allergic to lanreotide, somatostatin, or medicines of the same family (somatostatin analogs) or to any of the other components of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Myrelez:
- If you are diabetic, as lanreotide may affect your blood sugar levels. Your doctor will check your blood sugar levels and may modify your antidiabetic treatment while you are receiving lanreotide.
- If you have gallstones, as lanreotide may favor the formation of gallstones in the gallbladder. In this case, you may need to undergo periodic checks. Your doctor may decide to suspend treatment with lanreotide if complications arise from gallstones.
- If you have thyroid problems, as lanreotide may slightly decrease your thyroid function.
- If you suffer from cardiac problems, as treatment with lanreotide may cause sinus bradycardia (decreased heart rate). Caution should be exercised when initiating treatment with lanreotide in patients with bradycardia.
Talk to your doctor or pharmacist if you have any of the above before using Myrelez.
Children
Myrelez is not recommended for use in children.
Other medicines and Myrelez
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
You should be careful when taking Myrelez in combination with:
- Cyclosporin(a medicine that reduces immune reactions; it is usually used after a transplant or in case of autoimmune disease).
- Bromocriptine(a dopamine agonist, used in the treatment of certain types of brain tumors and Parkinson's disease or to prevent lactation after childbirth).
- Medicines that induce bradycardia(medicines that decrease heart rate, such as beta-blockers).
Your doctor will decide if adjustments are needed in the dose of these medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Lanreotide should only be administered if it is really necessary.
Driving and using machines
It is unlikely that treatment with Myrelez will affect your ability to drive or use machines, but you may experience side effects such as dizziness. If you do, be careful when driving or using machines.
3. How to use Myrelez
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Recommended dose
Treatment of acromegaly
The recommended dose is one injection every 28 days. Your doctor may adjust the dose of your injection using one of the three available doses of Myrelez (60, 90, or 120 mg).
If you are well-controlled with your treatment, your doctor may recommend changing the frequency of your Myrelez 120 mg injections to one injection every 42 or 56 days. Any change in dose will depend on the symptoms you experience and how you respond to the medicine.
Your doctor will also decide on the duration of treatment.
Relief of symptoms (such as hot flashes and diarrhea) associated with neuroendocrine tumors
The recommended dose is one injection every 28 days. Your doctor may adjust the dose of your injection using one of the three available doses of Myrelez (60, 90, or 120 mg).
If you are well-controlled with your treatment, your doctor may recommend changing the frequency of your Myrelez 120 mg injections to one injection every 42 or 56 days.
Your doctor will also decide on the duration of treatment.
Treatment of tumors of the intestine and pancreas, called gastroenteropancreatic neuroendocrine tumors or GEP-NETs. It is used when these tumors are advanced and cannot be removed by surgery.
The recommended dose is 120 mg every 28 days. Your doctor will decide on the duration of treatment with Myrelez for tumor control.
Method of administration
Myrelez should be administered by deep subcutaneous injection.
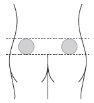
The injection should be administered in the upper outer quadrant of the buttock by a healthcare professional or by a caregiver (family member or friend) who has received adequate training.
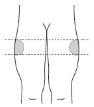
If, after being taught, you administer the injection yourself, the injection should be given in the upper outer thigh.
Your doctor should decide whether you should self-administer the medicine or have it administered by someone else who has been instructed to do so.
INSTRUCTIONS FOR USE
The following instructions explain how to inject Myrelez. Please read all the instructions carefully before starting the injection of the product. |
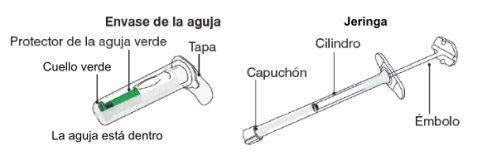
The content of the pre-filled syringe is a semi-solid phase with a gelatinous appearance, with viscous characteristics and a color that varies from white to pale yellow. The supersaturated solution may also contain microbubbles that can clear during injection. These differences are normal and do not interfere with the quality of the product. | |
B1. Remove Myrelez from the refrigerator 30 minutes before administration. Keep the laminated pouch closed until just before injection. B2. Before opening the pouch, check that it is intact and that the medicine has not expired. The expiration date is printed on the outer box and on the pouch. Do not use the pre-filled syringe if the product has expired or the pouch is damaged. B3. Wash your handswith soap and dry them well before starting. B4. Make sure you have a clean surface to prepare the injection. B5. Choose the injection site: see below. B6. Make sure to clean the injection site. B7. Tear open the pouch and remove the pre-filled syringe. | |
| |
| If a healthcare professional or a trained caregiver is administering the injection:use the upper outer quadrant of the buttock. |
| If you are administering the injection yourself:use the upper outer thigh. |
Alternate the injection sitebetween the left and right side each time you receive an injection of Myrelez. | |
| |
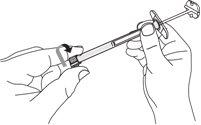
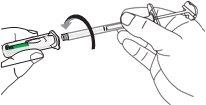
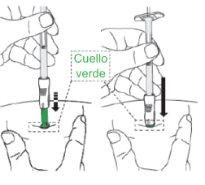
| C1: Remove the syringe cap.
|
| C2: Open the needle container
|
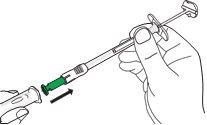
| C3: Insert the syringe into the needle container.
Important: Firmly adjust the syringe so that the medicine does not leak. |
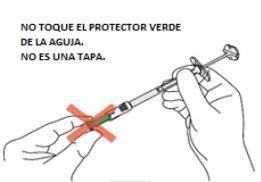
| C4: Remove the needle from the container.
Caution: From this step on, the needle is partially exposed.
|
| |
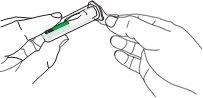
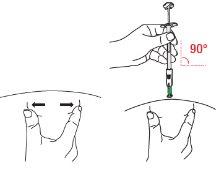
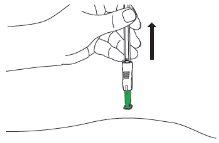
| D1: Position the syringe
|
| D2: Insert the needle
|
| D3: Press the plunger
|
| |
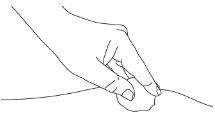
| E1: Remove from the skin
|
| E2: Press gently
|
| E3: Dispose of the syringe
|
If you use more Myrelez than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you have injected or been administered too much Myrelez, you may experience additional or more severe side effects (see section 4 "Possible side effects").
If you forget to use Myrelez
As soon as you realize you have forgotten an injection, consult your doctor and they will decide when you should receive the next injection. Do not self-inject additional injections to make up for missed injections without consulting your healthcare professional.
If you interrupt treatment with Myrelez
Interrupting treatment with Myrelez for more than one dose or prematurely ending treatment may affect the efficacy of the treatment. Consult your doctor before interrupting treatment.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Tell your doctor immediately if you notice any of the following adverse effects:
- Being thirstier or more tired than usual and having a dry mouth. These can be signs that you have high blood sugar levels or are developing diabetes.
- Feeling hungry, trembling, increased sweating, or a feeling of confusion. These can be signs of low blood sugar levels.
The frequency of these secondary effects is frequent, they can affect up to 1 in 10 people.
Tell your doctor immediately if you notice that:
- Your face turns red or swollen or you get spots or a rash.
- You feel pressure in your chest, have difficulty breathing, or wheezing.
- You feel dizzy, possibly as a result of a drop in blood pressure.
These symptoms can be the result of an allergic reaction.
The frequency of these secondary effects is unknown; it cannot be estimated from the available data.
Other Adverse Effects
Tell your doctor or pharmacist immediately if you experience any of the following adverse effects.
The most frequent adverse effects are gastrointestinal disorders, bile duct problems, and injection site reactions. The adverse effects that may occur with Myrelez are listed below according to their frequencies.
Very frequent: can affect more than 1 in 10 people:
- Diarrhea, soft stools, abdominal pain
- Gallstones and other bile duct disorders. You may have symptoms such as severe and sudden abdominal pain, high fever, jaundice (yellowing of the skin and whites of the eyes), chills, loss of appetite, itching of the skin
Frequent: can affect up to 1 in 10 people:
- Weight loss
- Lack of energy
- Slow heartbeats
- Feeling very tired
- Decreased appetite
- Feeling weak
- Excess fat in the stool
- Feeling dizzy, having a headache
- Hair loss or decreased body hair
- Pain in the muscles, ligaments, tendons, and bones
- Injection site reactions, such as pain and hardening of the skin
- Anomalies in liver and pancreas test results and changes in blood sugar levels
- Nausea, vomiting, constipation, gas, bloated stomach, or discomfort, indigestion
- Bile duct dilation (enlargement of the bile ducts between the liver and the gallbladder and the intestine). You may have symptoms such as stomach pain, nausea, jaundice, and fever
Uncommon: can affect up to 1 in 100 people:
- Hot flashes
- Difficulty sleeping
- Change in stool color
- Changes in blood test results for sodium and alkaline phosphatase levels
Frequency not known: frequency cannot be estimated from the available data:
- Sudden, severe pain in the lower abdomen. This can be a sign of pancreatitis (inflammation of the pancreas).
- Redness, pain, heat, and swelling at the injection site that may feel filled with fluid when pressed, fever. This can be a sign of an abscess.
- Sudden, severe pain in the upper right or central abdomen, which may extend to the shoulder or back, abdominal tenderness, nausea, vomiting, and high fever. This can be a sign of cholecystitis (inflammation of the gallbladder).
- Pain in the upper right abdomen, fever, chills, yellowing of the skin and eyes (jaundice), nausea, vomiting, clay-colored stools, dark urine, fatigue. These can be signs of cholangitis (inflammation of the bile duct).
Since lanreotide can alter your blood sugar levels, your doctor may want to monitor your blood sugar levels, especially at the start of treatment.
Similarly, as bile duct disorders can occur with this type of medication, your doctor may want to monitor your bile ducts at the start of treatment and from time to time once treatment has begun.
Tell your doctor or pharmacist if you experience any of the above-mentioned adverse effects.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Myrelez
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and label after "EXP". The expiration date is the last day of the month indicated.
The product should be administered immediately after opening the protective aluminum bag.
Store in the refrigerator (between 2°C and 8°C) in the original packaging to protect it from light.
The product can be stored again in the refrigerator (the number of temperature changes should not exceed three) for storage and later use, provided it has been stored in the sealed bag at a maximum temperature of 40°C for a maximum of 72 hours in total.
Each syringe is individually packaged.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Myrelez
- The active ingredient is lanreotide (60 mg, 90 mg, or 120 mg).
- The other ingredients are water for injectable preparations and glacial acetic acid (for pH adjustment).
Appearance of the Product and Package Contents
Myrelez is a viscous injectable solution, contained in a 0.5 ml semi-transparent plastic syringe and accompanied by a single-use needle with a safety device. Myrelez is presented in a semi-solid formulation of light yellow color.
Each pre-filled syringe is packaged in an aluminum bag and a cardboard box.
Box with a 0.5 ml syringe and a co-packaged safety needle (1.2 mm x 20 mm).
Multiple packaging with three boxes, each containing a 0.5 ml syringe with a co-packaged safety needle (1.2 mm x 20 mm).
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Amdipharm Limited
3 Burlington Road
Dublin 4,
Ireland
Manufacturer
Pharmathen S.A
Dervenakion 6,
Pallini Attiki, 15351,
Greece
Pharmathen International S.A
Industrial Park Sapes,
Rodopi Prefecture, Block No 5,
Rodopi 69300,
Greece
Local Representative:
Advanz Pharma Spain S.L.U.
Paseo de la Castellana 135, 7th floor
28046 Madrid (Spain)
Tel. +34 900 834 889
Date of the Last Revision of this Prospectus:October 2024.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MYRELEZ 120 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 120 mgActive substance: lanreotideManufacturer: Sun Pharmaceutical Industries (Europe) B.V.Prescription requiredDosage form: INJECTABLE, 60 mgActive substance: lanreotideManufacturer: Sun Pharmaceutical Industries (Europe) B.V.Prescription requiredDosage form: INJECTABLE, 90 mgActive substance: lanreotideManufacturer: Sun Pharmaceutical Industries (Europe) B.V.Prescription required
Online doctors for MYRELEZ 120 mg Injectable Solution in Pre-filled Syringe
Discuss questions about MYRELEZ 120 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions