
How to use Mirelez
Package Leaflet: Information for the User
Myrelez, 60 mg, solution for injection in a pre-filled syringe
Myrelez, 90 mg, solution for injection in a pre-filled syringe
Myrelez, 120 mg, solution for injection in a pre-filled syringe
Lanreotide
Read all of this leaflet carefully before using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- 1. What Myrelez is and what it is used for
- 2. What you need to know before you use Myrelez
- 3. How to use Myrelez
- 4. Possible side effects
- 5. How to store Myrelez
- 6. Contents of the pack and other information
1. What Myrelez is and what it is used for
Myrelez contains the active substance lanreotide, which belongs to a group of medicines called somatostatin analogues. It is similar to another substance (hormone) called somatostatin. Lanreotide reduces the activity of certain hormones such as growth hormone (GH) and insulin-like growth factor (IGF-1) and inhibits the release of certain gastrointestinal hormones and intestinal secretion. It also has an effect on certain advanced types of tumors (called neuroendocrine tumors) that occur in the gut and pancreas, by inhibiting or slowing their growth.
What Myrelez is used for:
- treatment of acromegaly (a condition where the body produces too much growth hormone);
- relief of symptoms such as hot flashes and diarrhea that sometimes occur in patients with neuroendocrine tumors (NETs);
- treatment and inhibition of growth of certain advanced tumors that occur in the gut and pancreas, called gastroenteropancreatic neuroendocrine tumors (GEP-NETs) - when they cannot be removed by surgery.
2. What you need to know before you use Myrelez
When not to use Myrelez:
- if you are allergic (hypersensitive) to lanreotide, somatostatin, or other medicines in the same group (somatostatin analogues), or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Myrelez, you should discuss with your doctor, pharmacist, or nurse:
- if you have diabetes, as lanreotide can cause fluctuations in blood sugar levels. During treatment with Myrelez, your doctor may recommend checking your blood sugar levels and possibly changing your diabetes treatment plan;
- if you have been diagnosed with gallstones, as Myrelez can promote the formation of gallstones. In this case, periodic examination is recommended. If complications of gallstones occur, your doctor may decide to stop treatment with lanreotide.
- if you have any thyroid function disorders, as lanreotide may slightly disrupt thyroid function;
- if you have heart function disorders, as Myrelez may cause sinus bradycardia (slow heart rate). Caution should be exercised when starting treatment with Myrelez in patients with bradycardia.
If you have any of the above conditions, you should discuss them with your doctor or pharmacist before starting treatment with Myrelez.
Children
Myrelez is not recommended for use in children.
Myrelez and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or might take. Be cautious when taking the following medicines:
- cyclosporine(an immunosuppressant used after transplantation or in autoimmune diseases);
- bromocriptine(a dopamine receptor agonist used to treat pituitary tumors, Parkinson's disease, or to inhibit lactation);
- medicines that cause bradycardia(medicines that slow heart rate, such as beta-blockers).
Your doctor may consider adjusting the dosage of these medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Myrelez will only be used if the potential benefit justifies the potential risk to the baby.
Driving and using machines
It is unlikely that Myrelez will affect your ability to drive or use machines, but during treatment with Myrelez, side effects such as dizziness may occur. If you experience such symptoms, be cautious when driving or using machines.
3. How to use Myrelez
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
Recommended dose
Treatment of acromegaly
The recommended dose is one injection of the medicine every 28 days. The dose of Myrelez used for injection will be chosen by your doctor from the three available strengths of Myrelez (60, 90, 120 mg). If the desired response is achieved, your doctor may recommend changing the frequency of injections of Myrelez 120 mg to one injection every 42 or 56 days. Any dose changes will depend on your symptoms and response to treatment. Your doctor will also decide on the duration of treatment.
Relief of symptoms (such as hot flashes and diarrhea) associated with neuroendocrine tumors
The recommended dose is one injection of the medicine every 28 days. The dose of Myrelez used for injection will be chosen by your doctor from the three available strengths of Myrelez (60, 90, 120 mg). If the desired response is achieved, your doctor may recommend changing the frequency of injections of Myrelez 120 mg to one injection every 42 or 56 days. Your doctor will also decide on the duration of treatment.
Treatment of advanced tumors that occur in the gut and pancreas, called gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Myrelez is used when these tumors cannot be removed by surgery
The recommended dose is 120 mg every 28 days. Your doctor will decide on the duration of treatment with Myrelez to inhibit tumor growth.
Method of administration
Myrelez should be administered by deep subcutaneous injection.
INSTRUCTIONS FOR USE
A. Contents of the box
Below is a description of how to perform the injection of Myrelez. Read the instructions carefully before performing the injection.
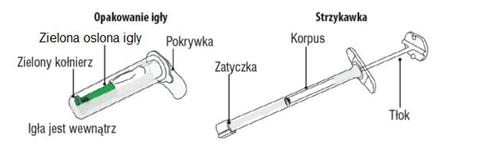
The contents of the pre-filled syringe are a semi-solid phase with a gel-like appearance, sticky properties, and a color ranging from white to light yellow. The supersaturated solution may also contain microbubbles, which may disappear during injection. These differences are normal and do not affect the quality of the product.
B. Before starting
B1. Remove Myrelez from the refrigerator 30 minutes before performing the injection. The laminated bag should be opened just before injection.
B2. Before opening the bag, check the integrity of the packaging and the expiration date of the medicine. The expiration date is printed on the bag and the box. – Do not use the medicine after the expiration date or if the bag is damaged.
B3. Before starting, wash your hands thoroughly with soap and dry them.
B4. Make sure the surface for preparing the injection is clean.
B5. Choose the injection site – the sites are shown below.
B6. Remember to clean the injection site.
B7. Tear open the bag and remove the pre-filled syringe.
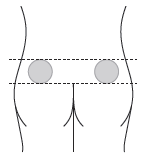
When injecting another person: inject into the upper outer quadrant of the buttock.
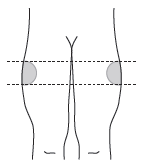
When self-injecting: inject into the upper outer part of the thigh. The injection site should be changed with each injection, alternating sides.
C. Preparing the syringe
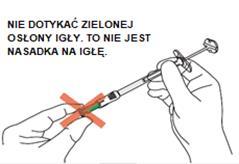
C1: Remove the cap from the syringe
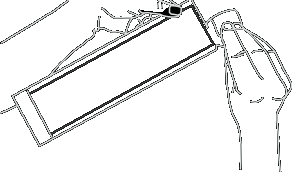
Hold the syringe body firmly with one hand (not the plunger). With the other hand, remove the cap by twisting it.
C2: Open the needle packaging.
Hold the needle packaging and pull off the cover. Note: Do not touch the open end of the needle packaging. It must remain clean.
C3: Insert the syringe into the open end of the needle packaging.
Hold the needle packaging with one hand. With the other hand, hold the syringe body firmly (not the plunger) and twist until the syringe and needle are fully locked.


The syringe and needle are fully locked when they can no longer be twisted.
Important: Make sure to tighten the syringe securely to avoid leakage of the medicine.
C4: Remove the needle from the packaging
Hold the syringe body firmly (not the plunger). Remove the needle straight from the packaging without twisting or turning it, to ensure the syringe is properly connected to the safety needle. Note: From this stage, the needle is partially exposed.
D. Performing the injection

D1: Position the syringe
The injection sites are shown in section B. Stretch the skin around the injection site with your thumb and index finger, so it is flat. With your other hand, hold the lower part of the syringe body (not the plunger). Position the syringe at a 90° angle to the skin.
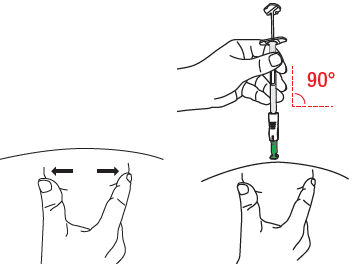
D2: Insert the needle
Without creating a fold in the skin or squeezing it at the injection site, firmly press the needle into the skin. The green needle shield will retract, and the safety mechanism will be activated.
Continue until only the collar of the green needle shieldis visible.
Do notpush the plunger at this stage. In the next step, please hold the syringe in this position.
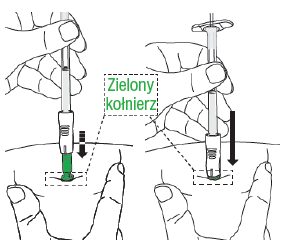
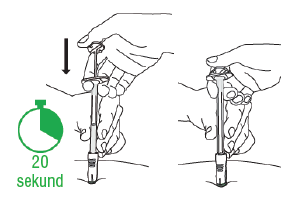
D3: Press the plunger
Move your hand from the skin to the plunger. Slowlypress the plunger until its top reaches the syringe body (it is easier to push the plunger with your dominant hand). This should take about 20 seconds.
E. Remove and dispose of the syringe
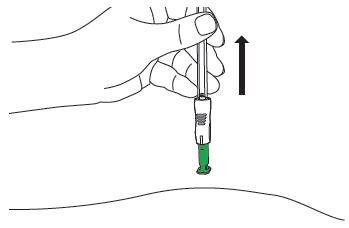
E1: Remove from the skin
Lift the syringe up and away from the patient's body. The green needle shield will cover the needle.
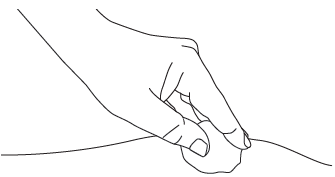
E2: Gently press
Gently press the injection site with a dry swab or sterile gauze to prevent bleeding. After administering the medicine, do notrub or massage the injection site.
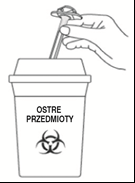
E3: Dispose of
Dispose of the used syringe and needle according to local regulations or your doctor's instructions. Needles are not reusable. Do notthrow the syringe or needle into household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
Using more Myrelez than you should
If you have injected more Myrelez than you should, tell your doctor. If you have injected more Myrelez than you should, there is a risk of additional or more severe side effects (see section 4. Possible side effects).
Forgetting to use Myrelez
If you forget to inject a dose, contact your doctor, who will inform you about the next injection. Do not inject a double dose to make up for a forgotten dose without consulting your doctor.
Stopping treatment with Myrelez
Missing more than one dose or stopping treatment with Myrelez early may affect the effectiveness of the treatment. Consult your doctor before stopping treatment with Myrelez. If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following side effects, contact your doctor immediately:
- increased thirst or fatigue and dry mouth. This may indicate that you have high blood sugar levels or are developing diabetes;
- hunger, shakiness, sweating, or confusion - these may be symptoms of low blood sugar levels. These side effects are common, affecting up to 1 in 10 people.
Contact your doctor immediately if you notice:
- redness or swelling of the face, rash, or hives,
- chest tightness, difficulty breathing, or wheezing,
- fainting, which may be due to low blood pressure. These may be symptoms of an allergic reaction. The frequency of this side effect is not known; it cannot be estimated from the available data.
Other side effects
If you experience any of the following side effects, contact your doctor or pharmacist immediately.
The most common side effects expected with Myrelez include gastrointestinal disorders, gallbladder disorders, and reactions at the injection site. Below is a list of side effects associated with Myrelez, including their frequency.
Very common: may affect more than 1 in 10 people:
- diarrhea, loose stools, abdominal pain
- gallstones and gallbladder disorders, which may cause severe and sudden abdominal pain, high fever, jaundice (yellowing of the skin and eyes), chills, loss of appetite, and itching.
Common: may affect up to 1 in 10 people:
- weight loss,
- lack of energy,
- slow heart rate,
- feeling very tired,
- decreased appetite,
- general weakness,
- excess fat in the stool,
- dizziness and headache,
- hair loss or reduced body hair growth,
- muscle, tendon, or bone pain,
- reactions at the injection site such as pain or hardening of the skin,
- abnormal liver or pancreas function test results and changes in blood sugar levels,
- nausea, vomiting, constipation, gas, bloating, or abdominal discomfort, indigestion,
- dilation of the bile ducts (enlargement of the bile ducts between the liver and the gallbladder and intestine). This may cause abdominal pain, nausea, jaundice, and fever.
Uncommon: may affect up to 1 in 100 people:
- hot flashes,
- difficulty sleeping,
- change in stool color,
- changes in sodium and alkaline phosphatase levels shown in blood tests.
Frequency not known: frequency cannot be estimated from the available data:
- sudden, severe abdominal pain - may be a symptom of pancreatitis,
- abscess at the injection site, which may feel fluid-filled when pressed (redness, pain, warmth, and swelling, possibly with fever),
- gallbladder inflammation (cholecystitis) - may cause severe and sudden abdominal pain, high fever, jaundice (yellowing of the skin and eyes), chills, loss of appetite, and itching,
- abdominal pain, fever, chills, jaundice (yellowing of the skin and eyes), dark urine, fatigue - may be symptoms of bile duct inflammation (cholangitis).
Since lanreotide can cause fluctuations in blood sugar levels, your doctor may recommend regular blood sugar checks, especially at the start of treatment. Similarly, due to the possibility of gallbladder disorders during treatment with Myrelez, your doctor may recommend regular gallbladder checks at the start of treatment and at regular intervals thereafter. If you experience any of the above side effects, tell your doctor or pharmacist.
Reporting side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the national reporting system: [insert contact information]. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Myrelez
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and label after “EXP”. The expiry date refers to the last day of that month. After opening the protective aluminum bag, use the medicine immediately. Store Myrelez in a refrigerator (2°C - 8°C) in the original package to protect from light. After removal from the refrigerator, the product can be stored in the closed bag at room temperature (below 40°C) for a maximum of 72 hours, with a maximum of three temperature excursions. Each syringe is packaged separately. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Myrelez contains
- The active substance is lanreotide (in doses of 60 mg, 90 mg, or 120 mg)
- The other ingredients are water for injections and glacial acetic acid (to adjust pH)
What Myrelez looks like and contents of the pack
Myrelez is a viscous solution for injection in a pre-filled syringe with a capacity of 0.5 ml, to which a single-use needle safety device is attached. It has a semi-solid consistency and a color ranging from white to light yellow. Each pre-filled syringe is packaged in an aluminum bag and a cardboard box. Pack sizes: 1 box containing a 0.5 ml pre-filled syringe with one safety needle (1.2 mm x 20 mm), packaged together. 1 multipack with 3 boxes, each containing a 0.5 ml pre-filled syringe with one safety needle (1.2 mm x 20 mm), packaged together. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Amdipharm Limited
3 Burlington Road
Dublin 4
Ireland
e-mail: [email protected]
Amdipharm Limited is part of the ADVANZ PHARMA group.
Manufacturer:
Pharmathen S.A.
Dervenakion 6
Pallini Attiki, 15351
Greece
Pharmathen International S.A.
Industrial Park Sapes
Rodopi Prefecture, Block No 5
Rodopi 69300
Greece
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria
Mytolac 60mg Injektionslösung in einer Fertigspritze
Mytolac 90mg Injektionslösung in einer Fertigspritze
Mytolac 120mg Injektionslösung in einer Fertigspritze
Belgium
Mytolac 60mg solution injectable en seringue préremplie
Mytolac 90mg solution injectable en seringue préremplie
Mytolac 120mg solution injectable en seringue préremplie
Czech Republic
Mytolente
Denmark
Myrelez 60mg injektionsvæske, opløsning i fyldt injektionssprøjte
Myerlez 90mg injektionsvæske, opløsning i fyldt injektionssprøjte
Myrelez 120mg, injektionsvæske, opløsning i fyldt injektionssprøjte
Estonia
Myrelez
Finland
Myrelez 60mg injektioneste, liuos esitäytetyssä ruiskussa
Myrelez 90mg injektioneste, liuos esitäytetyssä ruiskussa
Myrelez 120mg injektioneste, liuos esitäytetyssä ruiskussa
France
Myrelez L.P. 60mg solution injectable à libération prolongée en seringue préremplie
Myrelez L.P. 90mg solution injectable à libération prolongée en seringue préremplie
Myrelez L.P. 120mg solution injectable à libération prolongée en seringue préremplie
Germany
Mytolac 60mg Injektionslösung in einer Fertigspritze
Mytolac 90mg Injektionslösung in einer Fertigspritze
Mytolac 120mg Injektionslösung in einer Fertigspritze
Greece
Myrelez 60mg ενέσιμο διάλυμα σε προγεμισμένη σύριγγα
Myrelez 90mg ενέσιμο διάλυμα σε προγεμισμένη σύριγγα
Myrelez 120mg ενέσιμο διάλυμα σε προγεμισμένη σύριγγα
Hungary
Mytolac 60mg oldatos injekció előretöltött fecskendőben
Mytolac 90mg oldatos injekció előretöltött fecskendőben
Mytolac 120mg oldatos injekció előretöltött fecskendőben
Ireland
Myrelez 60mg solution for injection in a prefilled syringe
Myrelez 90mg solution for injection in a prefilled syringe
Myrelez 120mg solution for injection in a prefilled syringe
Italy
Myrelez
Latvia
Myrelez 60mg šķīdums injekcijām pilnšļircē
Myrelez 90mg šķīdums injekcijām pilnšļircē
Myrelez 120mg šķīdums injekcijām pilnšļircē
Lithuania
Myrelez 60mg injekcinis tirpalas užpildytame švirkšte
Myrelez 90mg injekcinis tirpalas užpildytame švirkšte
Myrelez 120mg injekcinis tirpalas užpildytame švirkšte
Netherlands
Mytolac 60mg oplossing voor injectie in een voorgevulde spuit
Mytolac 90mg oplossing voor injectie in een voorgevulde spuit
Mytolac 120mg oplossing voor injectie in een voorgevulde spuit
Norway
Myrelez 60mg injeksjonsvæske, oppløsning i ferdigfylt sprøyte
Myrelez 90mg injeksjonsvæske, oppløsning i ferdigfylt sprøyte
Myrelez 120mg injeksjonsvæske, oppløsning i ferdigfylt sprøyte
Poland
Myrelez
Portugal
Mytolac 60mg solução injetável em seringa pré-cheia
Mytolac 90mg solução injetável em seringa pré-cheia
Mytolac 120mg solução injetável em seringa pré-cheia
Romania
Mytolac 60mg soluţie injectabilă în seringă preumplută
Mytolac 90mg soluţie injectabilă în seringă preumplută
Mytolac 120mg soluţie injectabilă în seringă preumplută
Slovakia
Mytolente 60 mg injekčný roztok naplnený v injekčnej striekačke
Mytolente 90 mg injekčný roztok naplnený v injekčnej striekačke
Mytolente 120 mg injekčný roztok naplnený v injekčnej striekačke
Spain
Myrelez 60mg Solucion inyectable en jeringa precargada
Myrelez 90mg Solucion inyectable en jeringa precargada
Myrelez 120mg Solucion inyectable en jeringa precargada
Date of last revision of the leaflet: 16-05-2025
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterPharmathen International S.A. Pharmathen S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MirelezDosage form: Solution, 60 mgActive substance: lanreotidePrescription requiredDosage form: Solution, 90 mgActive substance: lanreotidePrescription requiredDosage form: Solution, 120 mgActive substance: lanreotidePrescription required
Alternatives to Mirelez in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Mirelez in Spain
Online doctors for Mirelez
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Mirelez – subject to medical assessment and local rules.







