
MOVYMIA 20 micrograms/80 microliters Injectable Solution

How to use MOVYMIA 20 micrograms/80 microliters Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Movymia 20 micrograms/80 microliters solution for injection
Teriparatide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Movymia and what is it used for
- What you need to know before you use Movymia
- How to use Movymia
- Possible side effects
- Storage of Movymia
- Contents of the pack and other information
1. What is Movymia and what is it used for
Movymia contains the active substance teriparatide, which is used to increase bone strength and reduce the risk of fractures by stimulating bone formation.
Movymia is used for the treatment of osteoporosis in adults. Osteoporosis is a disease that causes your bones to wear down and become fragile. This disease is especially common in women after menopause, but it can also occur in men. Osteoporosis is also common in patients treated with medicines called corticosteroids.
2. What you need to know before you use Movymia
Do not use Movymia
- if you are allergic to teriparatide or any of the other ingredients of this medicine (listed in section 6).
- if you have high levels of calcium in your blood (pre-existing hypercalcaemia).
- if you have severe kidney problems.
- if you have ever had bone cancer or if other types of cancer have spread (metastasized) to your bones.
- if you have certain bone diseases. If you have a bone disease, consult your doctor.
- if you have high levels of alkaline phosphatase in your blood without apparent explanation, which could indicate that you have Paget's disease of the bone (a disease with abnormal bone changes). If you are not sure, consult your doctor.
- if you have received radiation therapy that may have affected your bones.
- if you are pregnant or breastfeeding.
Warnings and precautions
Movymia may increase calcium in your blood or urine.
Talk to your doctor before or while using Movymia:
- If you constantly feel nauseous, vomit, are constipated, feel weak or have muscle weakness, tell your doctor. These may be symptoms of too much calcium in your blood.
- If you have kidney stones or have had kidney stones.
- If you have kidney problems (moderate renal insufficiency), you should tell your doctor.
Some patients, after the first doses of Movymia, may feel dizzy or have an increased heart rate. For the first doses, use Movymia in a place where you can sit or lie down immediately if you feel dizzy.
The recommended treatment time of 24 months should not be exceeded.
Before inserting a cartridge into the Movymia Pen, note the batch number (Batch) of the cartridge and the date of the first injection on the cartridge box and provide this information when reporting any adverse reaction.
Movymia should not be used in adults who are still growing.
Children and adolescents
Movymia should not be used in children and adolescents (under 18 years of age).
Other medicines and Movymia
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
This is important because some medicines (e.g., digoxin/digitals, a medicine used to treat heart diseases) may interact with teriparatide.
Pregnancy and breastfeeding
Do not use Movymia if you are pregnant or breastfeeding. If you are a woman of childbearing age, you should use effective contraceptive methods during treatment with Movymia. If you become pregnant while using Movymia, treatment should be discontinued. Consult your doctor or pharmacist before using this medicine.
Driving and using machines
Some patients may feel dizzy after the injection of Movymia. If you feel dizzy, do not drive or use machines until you feel better.
Movymia contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose unit; it is essentially "sodium-free".
3. How to use Movymia
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The recommended dose is 20 micrograms (corresponding to 80 microliters) administered once a day by subcutaneous injection in the thigh or abdomen.
To help you remember to inject your medicine, inject it at the same time every day.
Movymia can be injected at mealtime. Inject Movymia every day for as long as your doctor prescribes it. The total duration of treatment with Movymia should not exceed 24 months. You should not receive more than one 24-month treatment cycle in your lifetime.
Your doctor may advise you to use Movymia with calcium and vitamin D. Your doctor will tell you how much to take each day.
Movymia can be administered with or without food.
The Movymia cartridges are designed for use exclusively with the reusable Movymia Pen multidose administration system and compatible pen needles. The pen and needles are not included with Movymia. However, for the start of treatment, a package with a cartridge and pen should be used, which contains a cartridge case and a Movymia Pen case.
The pen can be used with injection needles developed according to the ISO standard for pen needles with a gauge between 29 G and 31 G (diameter of 0.25 - 0.33 mm) and a length between 5 mm and 12.7 mm only for subcutaneous injection.
Before the first use, insert the cartridge into the pen. To use this medicine correctly, it is very important that you follow the detailed instructions for use of the pen provided with it.
Use a new injection needle for each injection to prevent contamination and safely discard the needle after use.
Never store the pen with the needle attached.
Never share your pen with others.
Do not use the Movymia Pen to inject any other medicine (e.g., insulin). The pen is designed for use with Movymia only.
Do not refill the cartridge.
Do not transfer the medicine to a syringe.
You should inject Movymia shortly after removing the pen with the cartridge inserted from the refrigerator. Put the pen with the cartridge inserted back in the refrigerator immediately after use. Do not remove the cartridge from the pen after each use. Keep it in the cartridge holder for the complete 28-day treatment period.
Preparing the pen for use
- To ensure correct administration of Movymia, always read the Instructions for Use of Movymia Pen, included in the pen case.
- Wash your hands before handling the cartridge or pen.
- Check the expiration date on the cartridge label before inserting it into the pen.
Make sure there are at least 28 days left until the expiration date. Insert the cartridge into the pen before the first use as detailed in the pen instructions. Note the batch number (Batch) of each cartridge and the first injection date on a calendar.
You should also note the first injection date on the Movymia package (see the space provided on the box: {First use:}).
- After inserting a new cartridge and before the first injection of that cartridge, prepare the pen according to the instructions provided. Do not prepare it again after the first dose.
Injecting Movymia
- Before injecting Movymia, clean the skin where you plan to inject it (thigh or abdomen) as your doctor has indicated.
- Gently pinch the cleaned skin and insert the needle perpendicular to the skin. Press the button and keep it pressed until the dose indicator returns to the starting position.
- After the injection, leave the needle in the skin for six seconds to ensure you receive the full dose.
- Once the injection is complete, place the needle protective cap on the pen needle; screw the cap counterclockwise to remove the needle from the pen. This will maintain the sterility of the remaining Movymia and prevent leaks from the pen. It will also prevent air from being introduced back into the cartridge and the needle from becoming clogged.
- Replace the cap on the pen. Leave the cartridge in the pen.
If you use more Movymia than you should
If you have accidentally administered more Movymia than prescribed, consult your doctor or pharmacist.
The effects that can be expected from an overdose include nausea, vomiting, dizziness, and headache.
If you forget to use Movymia
If you miss an injection or cannot inject your medicine at the usual time, do so as soon as possible on the same day. Do not use a double dose to make up for missed doses. Do not inject more than once on the same day.
If you stop treatment with Movymia
If you are thinking of stopping treatment with Movymia, please consult your doctor. Your doctor will advise and decide how long you should be treated with Movymia.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common side effects are pain in the limbs (very common, may affect more than 1 in 10 patients). Other common side effects (affecting up to 1 in 10 patients) are discomfort, headache, and dizziness. If you feel dizzy after an injection, sit or lie down until you feel better. If it does not improve, consult your doctor before continuing treatment. There have been cases of fainting after the use of teriparatide.
If you experience discomfort around the injection site such as redness of the skin, pain, swelling, itching, bruising, or slight bleeding (which may occur frequently), these should disappear within a few days or weeks. If not, tell your doctor.
Rare (may affect up to 1 in 1,000 patients), some patients may experience allergic reactions, which consist of difficulty breathing, swelling of the face, rash, and chest pain. These reactions usually occur shortly after injection. In rare cases, severe and potentially life-threatening allergic reactions, including anaphylaxis, may occur.
Other side effects are:
Common(may affect up to 1 in 10 patients):
- increased cholesterol levels in the blood
- depression
- neuropathic pain in the leg
- feeling of fainting
- feeling that everything is spinning
- irregular palpitations
- difficulty breathing
- increased sweating
- muscle cramps
- loss of energy
- fatigue
- chest pain
- low blood pressure
- heartburn (pain or burning sensation just below the breastbone)
- vomiting
- hiatus hernia (hernia of the tube that carries food to the stomach)
- low hemoglobin or low red blood cell count (anemia)
Uncommon(may affect up to 1 in 100 patients):
- increased heart rate
- abnormal heart sound
- shortness of breath
- hemorrhoids (piles)
- loss of urine
- increased need to urinate
- weight gain
- kidney stones
- pain in the muscles and joints. Some patients have had severe back cramps or pain and had to be hospitalized.
- increased calcium levels in the blood
- increased uric acid levels in the blood
- increased levels of an enzyme called alkaline phosphatase
Rare(may affect up to 1 in 1,000 patients):
- reduced kidney function, including renal failure
- swelling, mainly in the hands, feet, and legs
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Movymia
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the package and cartridge after CAD and EXP, respectively. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze. Keep the cartridge in the case to protect it from light.
You can use Movymia for 28 days after the first injection, provided the cartridge/pen with the cartridge inserted is stored in a refrigerator (between 2 °C and 8 °C).
Avoid placing the cartridge near the freezer compartment of the refrigerator to prevent freezing. Do not use Movymia if it has been frozen.
Each cartridge should be disposed of properly after 28 days of first use, even if it is not completely empty.
Movymia contains a clear and colorless solution. Do not use Movymia if it has solid particles or if the solution is cloudy or discolored.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Movymia Composition
- The active ingredient is teriparatide. Each 80 microliter dose contains 20 micrograms of teriparatide. A 2.4 ml cartridge contains 600 micrograms of teriparatide (corresponding to 250 micrograms per ml).
- The other components are: glacial acetic acid, mannitol, metacresol, sodium acetate trihydrate, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment), and water for injectable preparations (see section 2 "Movymia contains sodium").
Product Appearance and Container Contents
Movymia is a clear and colorless injectable solution. It is presented in a cartridge. Each cartridge contains 2.4 ml of solution sufficient for 28 doses.
Container sizes: 1 cartridge or 3 cartridges packaged in a sealed plastic tray with a lid and packaged in a box.
Container with cartridge and Movymia Pen: 1 Movymia cartridge packaged in a sealed plastic tray with a lid and packaged in a box, and 1 Movymia Pen packaged in a box.
Only some container sizes may be marketed.
Marketing Authorization Holder
STADA Arzneimittel AG
Stadastrasse 2-18
61118 Bad Vilbel
Germany
Manufacturer
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungary
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Laboratorio STADA, S.L.
Phone: +34 934738889
Date of Last Revision of this Leaflet:September 2021.
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu/
Detailed information about this product is also available by scanning the QR code below or the box with a smartphone. The same information is also available at the following URL: movymiapatients.com
Instructions for Use
Movymia Pen
Reusable injection pen for use with Movymia cartridges, for subcutaneous injection
When using the Movymia Pen, always follow the instructions given below and on the back.
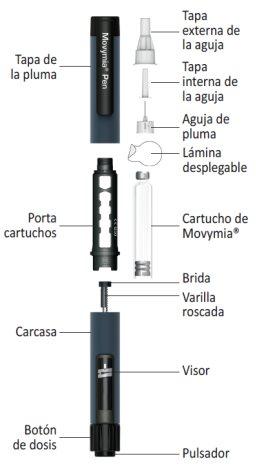
Parts of the Movymia Pen
Preparing the Pen - First Use / Cartridge Change
Follow the instructions each time you insert a new Movymia cartridge into the Movymia Pen. Do not repeat this step before each daily injection, as you will not have enough Movymia for 28 days.
Read the Movymia cartridge leaflet provided separately.
A: Remove the pen cap.
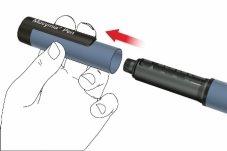
B: Turn the cartridge holder to remove it (bayonet fitting).

C: If you want to change the cartridge, remove the empty cartridge. Place a new Movymia cartridge in the cartridge holder with the cartridge's metal foldable cap first.
Record the date of the first injection of each new cartridge. This will help you know when the 28 daily doses per cartridge are finished.
D: Carefully push the threaded rod straight and to the stop with your finger. This is not necessary when the rod is already in the initial position, such as the first time it is used. The threaded rod cannot be fully inserted into the pen casing.
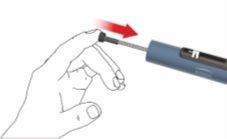
E: Attach the cartridge holder to the casing by turning it 90 degrees, until it stops.

F: Attach a new pen needle as follows:
- Remove the foil.
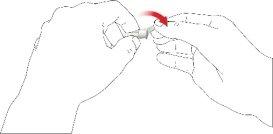
- Screw the pen needle clockwise onto the cartridge holder. Make sure the pen needle is properly seated and firmly supported on the cartridge holder.
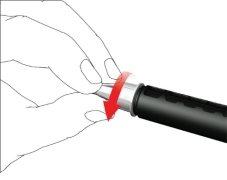
- Remove the outer needle cap and store it.
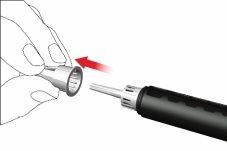
- Remove and discard the inner needle cap.
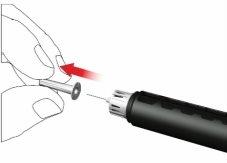
When attaching the needle, a few drops may escape; this is normal.
G: Priming
The pen must be primed and tested before inserting a new cartridge and before the first injection of each cartridge.
- Turn the dose button clockwise until you see the drop symbol in the dose window. Make sure the two lines of the indicator are aligned. During dose adjustment, the pen emits a click and offers a perceptible resistance.
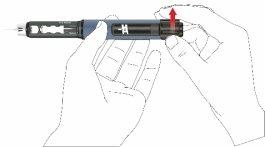
- Hold the pen with the needle pointing upwards.
- Press the button until the dose indicator returns to the initial position. Hold it pressed until some drops of the medication come out of the needle tip.
If not, repeat step G until you see some drops. Do not repeat step G more than four times; if necessary, follow the instructions in the Troubleshooting section on the back.
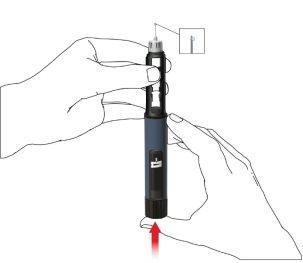
Administration using the Movymia Pen
Wash your hands carefully with soap to reduce the risk of infection.
Make sure you have:
- the Movymia Pen with the cartridge inserted
- a pen needle compatible with the pen
- a puncture-resistant container for used needles.
Do not usethe pen if the cartridge is cloudy or discolored or contains particles.
Read the Movymia cartridge leaflet provided separately.
- Place the pen needle
Use a new needle for each injection. Do not use the pen needle if the packaging is damaged or you did not open it.
Note:it is not necessary to change the needle when using immediately after preparing the pen. In this case, continue with the "2. Dose adjustment and injection" step.
- Remove the foil.
- Screw the pen needle clockwise onto the cartridge holder. Make sure the pen needle is properly seated and firmly supported on the cartridge holder.
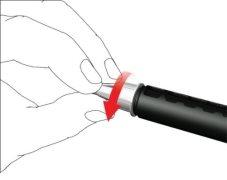
- Remove the outer needle cap and store it.
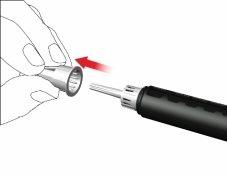
- Remove and discard the inner needle cap.
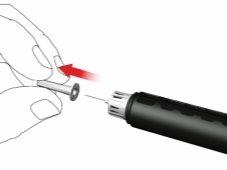
When attaching the needle, a few drops may escape; this is normal.
- Dose adjustment and injection
Warning: make sure to use the correct medication liquid. Check the cartridge label before inserting it into the cartridge holder.
- To adjust the fixed daily dose of 80 microliters, turn the dose button clockwise until it stops and cannot be turned further. Make sure the arrow symbol appears in the window and is aligned with the indicator line. During dose adjustment, the pen emits a click and offers a perceptible resistance. Do not try to force the dose button.
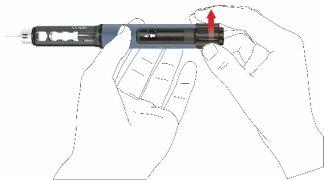
Note:if the cartridge contains less than 80 microliters, the dose button cannot be turned clockwise to the arrow symbol. In this case, remove the pen cap, change the cartridge, and perform a priming following the pen preparation steps.
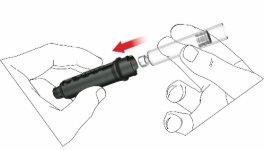
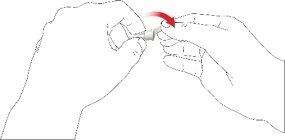
- Choose a suitable injection site and prepare the skin according to the doctor's recommendations. Carefully take a skin fold between the index and thumb fingers. Insert the pen needle straight and carefully into the skin, as shown in the illustration.
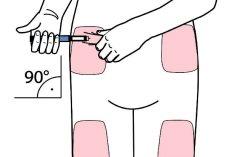
Warning:avoid bending or breaking the pen needle. Do not tilt the pen after inserting the needle into the skin. Tilting the pen can bend or break the needle. Broken needles can remain stuck in the skin. If a broken needle remains stuck in the skin, consult a doctor immediately.
- Press the button until the dose indicator returns to the initial position. Leave the needle in the skin fold for 6 seconds longer.
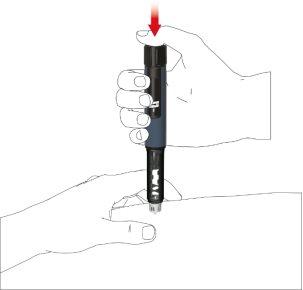
- Slowly remove the pen. Check if the window is in the initial position to ensure the full dose has been injected.

- Remove the needle from the pen
- Carefully place the outer needle cap over the pen needle.
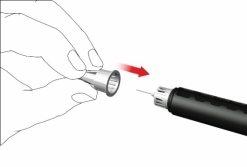
- Unscrew the needle cap counterclockwise to remove the needle from the pen. Dispose of it properly, for example in a puncture-resistant container for used needles.
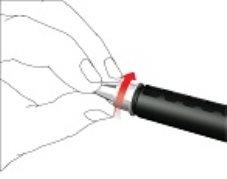
- Replace the pen cap
- Do not remove the cartridge from the Movymia Pen before it is empty.
- Replace the pen cap after each use.
- Put the Movymia Pen with the cartridge in the refrigerator at a temperature of 2 to 8 °C immediately after use.
Note for Healthcare Professionals
Local, healthcare professional, or institutional policies may replace the instructions regarding needle handling and disposal.
Additional Information
The reusable fixed-dose pen is designed for easy administration of Movymia to treat osteoporosis. Each Movymia cartridge contains 28 fixed doses of 80 microliters of Movymia.
Use the Movymia Pen only as directed by your doctor and according to the information in these instructions for use and the Movymia leaflet.
The Movymia Pen can be used by patients who self-inject and are over 18 years old, healthcare professionals, or third parties such as adult family members.
The Movymia Pen should not be used by blind or visually impaired patients without the help of a physically able person who is properly trained. Consult your doctor if you have hearing or handling problems.
If you have any questions about using the Movymia Pen, contact our Customer Serviceat any time.
Phone number: XXXXXXXXXXX
Email: XXXXXXXXXXX
Pen-compatible Needles
- Ypsomed mylife Clickfine, gauge 29 to 31 (diameter 0.25 - 0.33 mm) and length of 12, 10, 8 or 6 mm
- BD Micro-Fine Ultra needles, gauge 29 to 31 (diameter 0.25 - 0.33 mm) and length of 12.7, 8 or 5 mm
Pen needles from other manufacturers may be used according to the compatibility details indicated.
Pen needles must be used only onceand only one person should use the Movymia cartridge.
Storage and Care of the Movymia Pen
- Handle the pen carefully. Do not drop the pen and avoid hitting it against hard surfaces. Protect it from water, dust, and moisture.
- You can use a damp cloth to clean the Movymia Pen. Do not use alcohol, solvents, or cleaning agents. Do not submerge the Movymia Pen in water, as it may damage it.
- Do not use the Movymia Pen if it is damaged or if you have doubts about its proper functioning.
- Transport and store the Movymia Pen with the cartridge at the temperature indicated in the Movymia leaflet provided separately.
- Keep the Movymia Pen, cartridges, and needles out of the reach of children.
- Do not store the Movymia Pen with the needle attached, as it may cause air bubbles to form in the cartridge.
How to Dispose of the Movymia Pen and Used Accessories
The Movymia Pen has a lifespan of two years. Before disposing of the Movymia Pen, remove the needle and cartridge. Used needles and cartridges must be disposed of safely and separately. The Movymia Pen can be disposed of according to local authority instructions.
Warnings
Follow the instructions presented in these instructions for use. If these instructions are not followed, there is a risk of medication errors, inadequate dosing, disease transmission, or infection. If you have any health problems, seek medical attention immediately.
Warranty
The warranty covers material and manufacturing defects of the Movymia Pen for two years of use from the date of purchase. It is limited to replacing the pen. The warranty does not cover damage caused by:
- using cartridges that are not Movymia cartridges
- incorrect or negligent use, handling, or cleaning
- use contrary to the instructions for use
- using the pen with medical devices, accessories, or consumables other than those mentioned in these instructions for use
- falls, blows, application of force, contact with liquids
- other cases of exposure and wear that are not in accordance with the instructions for use.
Troubleshooting
If you have any questions about using the Movymia Pen, follow the instructions given in the table on the next page:
Question | Answer |
| A small air bubble will not affect the dose or cause damage. |
| Use another needle. If the second needle cannot be placed, contact Customer Service. |
| Use another needle. |
| Do not use that pen; contact Customer Service. |
| Change the needle and repeat the priming as indicated in the pen preparation sections "F" and "G". If medication still does not come out, do not use that pen; contact Customer Service. |
| The amount of Movymia remaining in the cartridge is less than 80 microliters. Change the cartridge and pen needle and perform a priming according to the pen preparation steps. |
| Do not repeat the injection on the same day. Use a new needle for the injection the next day. Adjust the dose and perform the injection as described in the "2. Dose adjustment and injection" section. If the window still does not return to the initial position after injection, do not use that pen; contact Customer Service. |
| Do not use that pen; contact Customer Service. |
How can I reset the dose button to the initial position? | Do not press the button. To reset the pen, simply turn the dose button counterclockwise to the initial position. |
Distributor:
XXXXXXXXXX
Importer:
Gedeon Richter Plc.
Gyömroi út 19-21
1103 Budapest
Hungary
Authorized Representative in the European Community:
Ypsomed Distribution GmbH
Warmbacher Strasse 80
79618 Rheinfelden
Germany
Legal Manufacturer:
Ypsomed AG
Brunnmattstrasse 6
3401 Burgdorf
Switzerland
CE 0123
Date of text revision:
- Country of registration
- Average pharmacy price252.16 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MOVYMIA 20 micrograms/80 microliters Injectable SolutionDosage form: INJECTABLE, 250 micrograms/mlActive substance: teriparatideManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, 250 µg/mlActive substance: teriparatideManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: INJECTABLE, 20 micrograms/80 microlitersActive substance: teriparatideManufacturer: Theramex Ireland LimitedPrescription required
Online doctors for MOVYMIA 20 micrograms/80 microliters Injectable Solution
Discuss questions about MOVYMIA 20 micrograms/80 microliters Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











