

Nasonex

Ask a doctor about a prescription for Nasonex

How to use Nasonex
Package Leaflet: Information for the User
Warning! Keep the leaflet. Information on the immediate packaging in a foreign language.
Nasonex, 50 µg/dose, nasal spray, suspension
Mometasone furoate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet
- 1. What is Nasonex and what is it used for
- 2. Important information before using Nasonex
- 3. How to use Nasonex
- 4. Possible side effects
- 5. How to store Nasonex
- 6. Contents of the pack and other information
1. What is Nasonex and what is it used for
What is Nasonex?
Nasonex nasal spray contains mometasone furoate, which belongs to a group of medicines called corticosteroids. Mometasone furoate administered intranasally relieves symptoms of inflammation (swelling and irritation of the nasal mucosa), sneezing, itching, and a stuffy nose, and reduces the amount of nasal discharge.
What is Nasonex used for?
Hay fever and perennial allergic rhinitis Nasonex is used to treat symptoms of hay fever (also known as seasonal allergic rhinitis) and perennial allergic rhinitis in adults and children aged 3 and older. Hay fever, which occurs at certain times of the year, is an allergic reaction caused by inhaling pollen from trees, grasses, weeds, as well as mold and fungal spores. Perennial allergic rhinitis occurs throughout the year, and symptoms can be caused by sensitivity to various factors, including house dust mites, animal dander (or shed skin), feathers, and some foods. Nasonex reduces swelling and irritation of the nasal mucosa, thereby relieving sneezing, itching, stuffy nose, or nasal discharge caused by hay fever or perennial allergic rhinitis. Nasal polyps Nasonex is used to treat nasal polyps in adults aged 18 and older. Nasal polyps are small growths on the nasal mucosa, usually occurring in both nostrils. Nasonex reduces inflammation in the nose, causing gradual reduction of nasal polyps, thereby reducing the feeling of a stuffy nose, which can make breathing difficult.
2. Important information before using Nasonex
When not to use Nasonex
Warnings and precautions
Before starting to use Nasonex, you should discuss it with your doctor or pharmacist:
While using Nasonex, you should discuss it with your doctor
If corticosteroids in the form of a nasal spray are taken in large doses for a long time, side effects may occur due to the absorption of the medicine into the body. If you experience itching or irritation of the eyes, your doctor may prescribe a different treatment with Nasonex. If you experience blurred vision or other vision disturbances, you should contact your doctor.
Children
If corticosteroids in the form of a nasal spray are taken in large doses for a long time, they may cause certain side effects, such as slowing down the growth rate in children. It is recommended that the growth of children receiving long-term treatment with nasal corticosteroids be regularly monitored, and if any changes are observed, the doctor should be informed.
Nasonex and other medicines
You should tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take, including those that are available without a prescription. If you are taking other corticosteroids orally or by injection to treat allergies, your doctor may advise you to stop taking them when you start using Nasonex. In some patients, after stopping oral or injectable corticosteroids, side effects such as joint or muscle pain, weakness, and depression may occur. Other allergic symptoms, such as itching, tearing of the eyes, or red and itchy spots on the skin, may also appear. You should contact your doctor if you experience any of these symptoms. Some medicines may enhance the effect of Nasonex, and your doctor may want to monitor your condition closely if you are taking such medicines (including certain HIV medicines: ritonavir, cobicistat).
Pregnancy and breastfeeding
There are no data or only limited data on the use of Nasonex in pregnant women. It is not known whether mometasone furoate passes into breast milk. If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Driving and using machines
There are no data on the effect of Nasonex on the ability to drive and use machines.
Nasonex contains benzalkonium chloride
Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
3. How to use Nasonex
Nasonex should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist. The nasal spray should not be used more frequently, for a longer period, or in larger doses than prescribed by your doctor.
Treatment of hay fever and perennial allergic rhinitis
Use in adults and children over 12 years of age
Typically, two sprays are used in each nostril once a day.
- After improvement, your doctor may recommend reducing the dose.
- If you do not feel any improvement, you should contact your doctor, who may recommend increasing the dose: the maximum daily dose is four sprays in each nostril once a day.
Use in children aged 3 to 11 years
Typically, one spray is used in each nostril once a day.
In some patients, relief of symptoms occurs within 12 hours of administration of the first dose of Nasonex; however, full benefits of treatment may not be observed until after 2 days of treatment. Therefore, you should continue to use the spray regularly to achieve full benefits from treatment. If you have severe symptoms of hay fever, it may be necessary to start using Nasonex a few days before the expected start of the pollen season. This will help prevent the occurrence of hay fever symptoms.
Nasal polyps
Use in adults over 18 years of age
Typically, two sprays are used in each nostril once a day.
- If after 5 to 6 weeks of treatment with Nasonex no improvement occurs, your doctor may recommend increasing the dose to two sprays in each nostril twice a day. After improvement, your doctor may recommend reducing the dose of the medicine.
- If after 5 to 6 weeks of treatment with Nasonex twice a day no improvement occurs, you should contact your doctor.
Preparing the nasal spray for use
Nasonex nasal spray contains a protective cap that protects the tip of the actuator and prevents it from becoming contaminated. Remember to remove it before use and replace it after use. Before first use, check the functioning of the spray by pressing the pump 10 times until a fine mist is produced:
- 1. Gently shake the bottle.
- 2. Place your index and middle fingers on either side of the actuator and your thumb under the bottle. Do notpuncture the nasal spray actuator.
- 3. With the actuator pointing away from you, press your fingers together to release 10 sprays, until a fine mist is produced. If the nasal spray has not been used for 14 days or longer, press the pump 2 times, until a fine mist is produced, before next use.
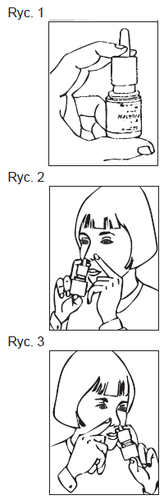
How to use the nasal spray
- 1. Shake the bottle gently and remove the protective cap. (Figure 1)
- 2. Gently blow your nose.
- 3. Close one nostril and insert the actuator into the other nostril, as shown in the picture. (Figure 2) Tilt your head slightly forward, keeping the bottle upright.
- 4. Start to breathe in gently and slowly through your nose, while spraying the medicine (in the form of a fine mist) into your nose, pressing DOWN ONCE with your fingers.
- 5. Breathe out through your mouth. Repeat the steps described in point 4 to administer a second spray into the same nostril, if necessary.
- 6. Remove the actuator from your nostril and breathe out through your mouth.
- 7. Repeat the steps described in points 3 to 6 to administer the spray into the other nostril. (Figure 3)
After use, gently wipe the actuator with a clean tissue or cloth and replace the protective cap.
Page 4 of 7 Cleaning
- It is important to regularly clean the nasal spray bottle, as it may not work properly otherwise.
- Remove the protective cap and gently pull off the actuator.
- Wash the actuator and protective cap in warm water, then rinse under running water.
- Do not attempt to unblock the actuator by puncturing it with a needle or other sharp object, as this will damage the actuator and the correct dose of the medicine will not be delivered.
- Leave the protective cap and actuator in a warm place to dry.
- Replace the actuator on the bottle, then put on the protective cap.
- After cleaning, check that the actuator is working properly and spray the medicine 2 times.
Using a higher dose of Nasonex than recommended
If you accidentally use a higher dose of Nasonex than recommended, you should consult your doctor. If steroids are used for a long period or in large doses, they may have an adverse effect on your hormones. In children, they may affect growth and development.
Missing a dose of Nasonex
If you forget to use the nasal spray at the right time, you should use it as soon as you remember, and then continue treatment at the usual time. Do not use a double dose to make up for a missed dose.
Stopping treatment with Nasonex
In some patients, relief of symptoms occurs within 12 hours of administration of the first dose of Nasonex; however, full benefits of treatment may not be observed until after 2 days of treatment. It is very important that you use the nasal spray regularly. You should not stop treatment, even if you feel better, unless your doctor advises you to do so. If you have any further doubts about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Nasonex can cause side effects, although not everybody gets them. After using this medicine, immediate allergic reactions (hypersensitivity) may occur. These reactions can be severe. You should stop using Nasonex and seek medical help immediately if you experience symptoms such as:
- swelling of the face, tongue, or throat
- difficulty swallowing
- hives
- wheezing or difficulty breathing
If corticosteroids in the form of a nasal spray are taken in large doses for a long time, side effects may occur due to the absorption of the medicine into the body. Other side effects Page 5 of 7 Most people using nasal sprays do not report any side effects. However, some people using Nasonex or other corticosteroids in the form of a nasal spray have reported the following side effects:
- headache
- sneezing
- nasal bleeding [very common (may occur in more than 1 in 10 people) in patients with nasal polyps who used two sprays of Nasonex in each nostril twice a day]
- irritated nose or sore throat
- ulceration of the nasal mucosa
- upper respiratory tract infection
Frequency not known (frequency cannot be estimated from the available data):
- increased intraocular pressure (glaucoma) and/or cataracts causing vision disturbances,
- damage to the nasal septum separating the nostrils
- disturbances of taste and smell
- breathing difficulties and/or wheezing
- blurred vision
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products Al. Jerozolimskie 181C PL-02 222 Warsaw Tel.: + 48 22 49 21 301 Fax: + 48 22 49 21 309 Website: https://smz.ezdrowie.gov.pl. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Nasonex
- Do not store above 25°C. Do not freeze.
- Keep the medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
- The bottle should be used within 2 months of opening. Only open one bottle at a time.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Nasonex contains
Page 6 of 7
- The active substance of Nasonex is mometasone furoate. One spray contains 50 micrograms of mometasone furoate in the form of a monohydrate.
- The other ingredients of the medicine are: dispersible cellulose (microcrystalline cellulose and sodium carmellose), glycerol, citric acid monohydrate, sodium citrate, polysorbate 80, benzalkonium chloride, purified water.
- This medicine contains 0.02 mg of benzalkonium chloride in one spray.
What Nasonex looks like and contents of the pack
Nasonex is a nasal spray, suspension. Each bottle contains 140 doses of the medicine. For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Belgium, the country of export:
Organon Belgium, Handelsstraat 31/Rue du Commerce 31, B-1000 Brussels, Belgium
Manufacturer:
NV Schering-Plough Labo, Industriepark 30, B-2220 Heist-op-den-Berg, Belgium
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź Belgian marketing authorization number: BE190854
Parallel import authorization number: 168/22
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria: Nasonex aquosum-Nasenspray Belgium, Croatia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Slovenia, Sweden, United Kingdom: Nasonex Bulgaria, Czech Republic, Romania, Slovakia: NASONEX Latvia: Nasonex 50 mikrogrami/deva deguna aerosols, suspensija Portugal: Nasomet Spain: NASONEX 50 microgramos suspensión para pulverización nasal Date of approval of the leaflet: 14.04.2022[Information about the trademark] Page 7 of 7
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Organon Belgium
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NasonexDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FARMEA US Pharmacia Sp. z o.o.Prescription not requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FarmeaPrescription requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: Adamed Pharma S.A. FarmeaPrescription not required
Alternatives to Nasonex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nasonex in Spain
Alternative to Nasonex in Ukraine
Online doctors for Nasonex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nasonex – subject to medical assessment and local rules.








