
METOTREXATE WYETH 2.5 mg TABLETS


How to use METOTREXATE WYETH 2.5 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Methotrexate Weekly Wyeth 2.5 mg Tablets
DO NOT EXCEED THE RECOMMENDED DOSE PRESCRIBED BY YOUR DOCTOR. THE DOSE FOR RHEUMATOID ARTHRITIS, JUVENILE IDIOPATHIC ARTHRITIS, PSORIASIS, PSORIATIC ARTHRITIS, REACTIVE ARTHRITIS, ACUTE LYMPHOBLASTIC LEUKEMIA, AND LYMPHOMAS IS ADMINISTERED ONCE A WEEK.DEATHS HAVE BEEN REPORTED WITH THE DAILY ADMINISTRATION OF THIS MEDICINE, INSTEAD OF WEEKLY. CONSULT YOUR DOCTOR OR PHARMACIST IF YOU ARE NOT SURE ABOUT THE QUANTITY AND FREQUENCY YOU SHOULD TAKE THIS MEDICINE.
Read the entire package leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Methotrexate Weekly Wyeth and what is it used for
- What you need to know before you start taking Methotrexate Weekly Wyeth
- How to take Methotrexate Weekly Wyeth
- Possible side effects
- Storage of Methotrexate Weekly Wyeth
- Package Contents and Additional Information
1. What is Methotrexate Weekly Wyeth and what is it used for
Methotrexate Weekly Wyeth belongs to a group of medicines called folinic acid antimetabolite analogues.
High-dose Methotrexate is indicated for the treatment of certain types of cancer, such as leukemias and lymphomas.
Low-dose Methotrexate is indicated for:
- Treatment of different types of arthritis, such as active and severe rheumatoid arthritis in adult patients, and active and severe polyarticular juvenile idiopathic arthritis, when the response to non-steroidal anti-inflammatory drugs (NSAIDs) has been inadequate.
- Treatment of psoriasis and psoriatic arthritis when other treatments have failed.
- Treatment of reactive arthritis when other treatments have failed.
2. What you need to know before starting to take Metotrexato semanal Wyeth
Before starting to use this medication, you should consult your doctor about the risks and benefits of treatment with metotrexato. It is very important that you use metotrexato exactly as your doctor has indicated. If you use metotrexato more frequently or in higher doses than indicated by your doctor, you may suffer from serious adverse reactions, including death.
Do not take MetotrexatosemanalWyeth
- If you are allergic to metotrexato or to any of the other components of this medication (included in section 6).
- If you have liver function disorders (hepatic insufficiency).
- If you have severe kidney function disorders (severe renal insufficiency).
- If you have liver damage due to excessive alcohol consumption (alcoholic hepatopathy), if you have chronic liver damage (chronic hepatopathy) or if you are an alcoholic.
- If you have altered blood levels of red blood cells, white blood cells, and platelets.
- If you suffer from any disease of the immune system (immunodeficiency syndromes).
- Severe, acute or chronic infections such as tuberculosis and HIV.
- If you have ulcers in the mouth, stomach, or intestine.
- If you are going to be vaccinated.
- If you are breastfeeding
In addition, in non-oncological indications (treatment not related to cancer)
- If you are pregnant (see section "Pregnancy, breastfeeding, and fertility");
Warnings and precautions
Important warning about the dose ofMetotrexato semanal Wyeth 2.5 mg tablets:
Take Metotrexato semanal Wyeth only once a week for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, psoriatic arthritis, reactive arthritis, acute lymphoblastic leukemia, and lymphomas.
Taking too much metotrexato can be fatal. Read section 3 of this prospectus carefully. If you have any questions, consult your doctor or pharmacist before taking this medication.
Consult your doctor, pharmacist, or nurse before starting to take this medication.
- Take Metotrexato semanal Wyeth once a week.
- Daily intake of the medication by mistake produces severe and life-threatening toxicity.
Your doctor will inform you of the benefits and risks of treatment with metotrexato, as well as the symptoms that may indicate possible toxicity due to the medication.
If you are in any of the following cases, consult your doctor before using this medication.
- If you develop signs or symptoms due to possible toxicity of the medication at the gastrointestinal and/or neuronal level, in the liver, kidney, lung, blood, or skin, consult your doctor.
- Follow the dose strictly as indicated by your doctor, for example, a dose prescribed weekly that is administered daily by mistake, may lead to toxicity, even fatal.
- Its use is not recommended in the treatment of neoplastic diseases in women of childbearing age, unless there is clear medical evidence that the expected benefits outweigh the risks.
- If you have folate deficiency as it could increase the toxicity to metotrexato.
- If you experience vomiting, diarrhea, or mouth inflammation (stomatitis), inform your doctor as you may become dehydrated. If this occurs, your doctor may interrupt treatment until you fully recover. Also, inform your doctor if you have peptic ulcers or any type of colitis.
- If you have alterations in blood levels of white blood cells, red blood cells, and platelets. Metotrexato can decrease white blood cells in the blood. If this occurs, you should take a series of precautions that include: avoiding contact with people with infections; consulting your doctor if you think you may have an infection due to the presence of fever or chills, cough, back pain, or difficulty urinating; consulting your doctor before undergoing dental interventions. Similarly, metotrexato can decrease the number of platelets in the blood necessary for coagulation. Therefore, it is essential that you consult your doctor in the presence of noticeable bruising or bleeding from the gums or nose, red spots on the skin, blood in the urine or black stools, and that you communicate to your dentist that you are being treated with metotrexato.
- If you are being treated with NSAIDs (non-steroidal anti-inflammatory drugs).
- If you have liver problems since metotrexato can cause acute hepatitis and chronic liver disorders. Moderate liver alterations may appear that require closer monitoring by the doctor but do not lead to the suspension of treatment. Alcohol, obesity, advanced age, or the use of products containing arsenic can increase the risk of liver problems.
- If you have kidney problems since metotrexato can cause kidney damage.
- If you suffer from any type of infectious process.
- If you are going to be vaccinated as it could produce a severe infection or the response to the vaccine could decrease.
- If during or after treatment, symptoms of toxicity at the level of the nervous system appear, such as headache, back pain, stiffness in the neck, fever, confusion, irritability, drowsiness, discoordination in movements, dementia, convulsions, transient blindness, abnormal reflexes, abnormal behavior, and localized alterations of movement and perception.
- If during treatment you experience dry cough, fever, chest pain, and/or difficulty breathing, inform your doctor.
- If during treatment or days later you develop skin alterations, consult your doctor. Skin lesions in patients with psoriasis can worsen due to exposure to sunlight. Previous skin lesions and sunburns may reappear with the use of metotrexato. Metotrexato can make the skin more sensitive to sunlight. Avoid intense sun and do not use tanning beds or ultraviolet lamps without medical advice. To protect your skin from intense sun, wear suitable clothing or use a sunscreen with a high protection factor.
- Acute pulmonary hemorrhage has been reported with metotrexato in patients with underlying rheumatological disease. If you observe blood when coughing or spitting, you should contact your doctor immediately.
- Metotrexato temporarily affects the production of sperm and eggs. Metotrexato can cause abortions and severe birth defects. If you are a woman, you should avoid pregnancy during treatment with metotrexato and for at least 6 months after the end of treatment with metotrexato. If you are a man, you should avoid having a child if you are being administered metotrexato at that time and for at least 3 months after finishing your treatment. See also the section "Pregnancy, breastfeeding, and fertility".
- If you, your partner, or your caregiver notice the appearance or worsening of neurological symptoms, such as general muscle weakness, vision alterations, changes in thinking, memory, and orientation that generate confusion and changes in personality, contact your doctor immediately because these can be symptoms of a rare and severe brain infection called progressive multifocal leukoencephalopathy (PML).
Recommended precautions and complementary tests
Even if metotrexato is used at low doses, serious adverse effects can occur. To detect them in time, your doctor should perform control tests and analytical tests.
Before starting treatment:
Before starting treatment, your blood will be analyzed to see if you have enough blood cells. Your blood will also be analyzed to check the functioning of your liver and to see if you have hepatitis. Additionally, serum albumin (a protein in the blood), hepatitis status (liver infection), and renal function will be checked. The doctor may also decide to perform other liver tests, some of which may involve images of your liver and others may require a small tissue sample from your liver to examine it more closely. Your doctor may also check if you have tuberculosis and take a chest X-ray or perform a respiratory function test.
During treatment:
Your doctor may perform the following tests:
- Examine the oral cavity and pharynx to detect changes in the mucosa, such as inflammation or ulceration;
- Blood tests/complete blood count with blood cell count and measurement of serum metotrexato levels;
- Blood tests to monitor liver function;
- Diagnostic imaging tests to monitor liver status;
- A small tissue sample taken from the liver to examine it more closely;
- Blood tests to monitor renal function;
- Monitoring of the respiratory tract and, if necessary, pulmonary function test.
If the results of any of these tests are remarkable, your doctor will adjust your treatment accordingly.
Elderly patients
Elderly patients treated with metotrexato should be closely monitored by a doctor so that possible adverse effects can be detected as soon as possible.
The deterioration of liver and kidney function related to age, as well as the low body reserves of folic acid in old age, require a relatively low dose of metotrexato.
Your doctor will regularly check your condition to see if the medication is having the expected effect.
Children, adolescents, and elderly people
Children, adolescents, and elderly people treated with metotrexato should be subject to close medical supervision to identify possible adverse effects as soon as possible.
The use of this medication is not recommended in children under 3 years of age because the experience with the use of this medication is insufficient in that age group
Other medicationsand MetotrexatosemanalWyeth
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
- Medications used to treat certain types of cancer, such as cisplatin, mercaptopurine, cytarabine, and L-asparaginase.
- Non-steroidal anti-inflammatory drugs, such as aspirin and other salicylates, and especially ketoprofen.
- Proton pump inhibitors (used to treat digestive system ulcers).
- Leflunomide (medication for the treatment of arthritis).
- Metamizol (synonyms novaminsulfon and dipyrone) (medication for intense pain and/or fever).
- Medications with high binding to plasma proteins (such as salicylates, phenylbutazone, phenytoin, sulfonamides, sulfonilureas, aminobenzoic acid, some antibiotics, and medications for the treatment of abnormal cholesterol and lipid levels such as cholestyramine).
- Probenecid (medication used to decrease uric acid levels).
- Antibiotics (ciprofloxacin, penicillins, sulfonamides, tetracyclines, chloramphenicol, pyrimethamine, trimethoprim/sulfamethoxazole) and broad-spectrum antibiotics that are not absorbed at the digestive level.
- Medications that produce liver toxicity (such as leflunomide, azathioprine, sulfasalazine, and retinoids).
- Theophylline (medication for the treatment of asthma).
- Vitamins or vitamin preparations that contain folic acid or its derivatives.
- Nitrous oxide (anesthetic).
- Amiodarone (medication for the treatment of cardiac disorders).
- Diuretics (such as triamterene).
Additionally, certain therapies may interact with metotrexato. This is the case of PUVA therapy (methoxsalen and ultraviolet light) in patients with psoriasis or a disease called mycosis fungoides, as well as radiotherapy.
During treatment with metotrexato, caution should be exercised when receiving a red blood cell transfusion.
Use of MetotrexatosemanalWyeth with food, beverages, and alcohol
While taking metotrexato, you should avoid consuming alcohol, as this could increase the likelihood of adverse effects, especially in the liver. You should avoid excessive consumption of coffee, caffeinated soft drinks, and black tea. Your doctor may indicate that you drink more liquids than usual. This will help you eliminate the medication and prevent kidney problems.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Consult your doctor or pharmacist before using any medication.
- Pregnancy:
Do not use this medication during pregnancy except if your doctor has prescribed it as oncological treatment (cancer treatment). Metotrexato can cause birth defects, harm the fetus, or cause abortions. It is associated with malformations of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that it is not administered to pregnant women or those who plan to become pregnant, unless it is used as oncological treatment.
In non-oncological indications (not related to cancer) in women of childbearing age, any possibility of pregnancy should be excluded, for example, through a pregnancy test, before starting treatment.
Do not use this medication if you are trying to become pregnant. You should avoid becoming pregnant while taking metotrexato and for at least 6 months after the end of treatment with metotrexato. To do this, you should ensure that you are using reliable contraceptive methods during this entire time (see also the section "Warnings and precautions").
If you become pregnant during treatment or suspect that you may be pregnant, consult your doctor as soon as possible. If you become pregnant during treatment, you should receive information about the risk of harmful effects on the child during treatment.
If you wish to become pregnant, consult your doctor, who may refer you to a specialist to inform you before the planned start of treatment.
- Breastfeeding:
Do not breastfeed your child during treatment, because metotrexato passes into breast milk. If the doctor considers it absolutely necessary to continue treatment with metotrexato during the breastfeeding period, you should stop breastfeeding.
- Male fertility:
Available data do not indicate a higher risk of malformations or abortions if the father takes a dose of metotrexato less than 30 mg/week. However, this risk cannot be completely ruled out, and there is no information regarding higher doses of metotrexato. Metotrexato can be genotoxic, which means it can cause genetic mutations. Metotrexato can affect sperm production, which is associated with the possibility of birth defects.
For this reason, you should avoid fathering a child or donating sperm during treatment with metotrexato and for at least 3 months after the end of treatment. Since treatment with metotrexato in higher doses commonly used in cancer treatment can cause infertility and genetic mutations, it is recommended that men treated with doses of metotrexato higher than 30 mg/week consider sperm conservation before starting treatment (see also the section "Warnings and precautions").
Driving and using machines
MetotrexatosemanalWyeth contains lactose
This medication contains lactose. If your doctor has indicated that you have intolerance to certain sugars, consult with them before taking this medication.
3. How to Take Metotrexato Weekly Wyeth
Follow exactly the administration instructions of this medication as indicated by your doctor or pharmacist and take only the number of tablets that have been prescribed for you to treat your disease. In case of doubt, consult your doctor or pharmacist again. If you use metotrexato more frequently or in higher doses than indicated by your doctor, you may suffer from serious adverse reactions, including death.
Recommended Dose:
Dose in arthritis, psoriasis, and reactive arthritis:
Take Metotrexato Weekly Wyeth 2.5 mg tablets only once a week.
The initial dose for rheumatoid arthritis in adults is 3 tablets (7.5 mg) once a week. According to the treatment evolution, your doctor may consider increasing the dose by 1 tablet every 6-8 weeks, up to a maximum dose of 8 tablets (20 mg) per week.
The recommended initial dose for juvenile idiopathic polyarticular arthritis is 10-15 mg/m2 once a weekadministered orally.
The initial dose in adults for psoriasis and psoriatic arthritis is 3 tablets (7.5 mg) once a week. According to the treatment evolution, your doctor may consider increasing the dose by 1 tablet every 4-6 weeks, up to a maximum dose of 10 tablets (25 mg) per week.
The recommended dose for reactive arthritis is 3 tablets (7.5 mg) once a week. According to the treatment evolution, your doctor may consider increasing the dose up to a maximum of 8 tablets (20 mg) per week.
Dose in leukemias and lymphomas:
Your doctor will indicate what dose you should take for your disease and when you should take it. Take exactly that dose.
It is possible that several months may pass before you can appreciate the complete benefits of this medication. Do not increase your dose or take this medication more frequently or for a longer time than prescribed. Your disease will not improve more quickly, and instead, you may increase your risk of experiencing adverse effects, which could be potentially fatal.
Graphic information on taking tablets in inflammatory indications for adults
Each package of Metotrexato Weekly Wyeth contains 24 tablets, which are presented in 2 blisters, each containing 12 tablets. Each tablet contains a dose of 2.5 mg of metotrexato. The following graphic schemes show the recommended amount of tablets to be taken for each of the inflammatory indicationsdescribed above. It is very important that you follow your doctor's instructions and take only the correct number of tablets that your doctor has prescribed for you to treat your disease. Remember that taking the tablets indicated by your doctor must be done 1 time per week. Please note the day chosen for weekly intake in the space provided for this purpose on the package.
RHEUMATOID ARTHRITIS, PSORIASIS, AND PSORIATIC ARTHRITIS
The initial dose in adults is 3 tablets (7.5 mg) once a week. Therefore, the package of Metotrexato Weekly Wyeth containing 24 tablets covers the treatment for 8 weeks, distributing the tablets as follows:
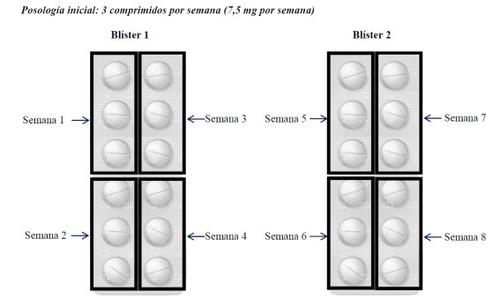
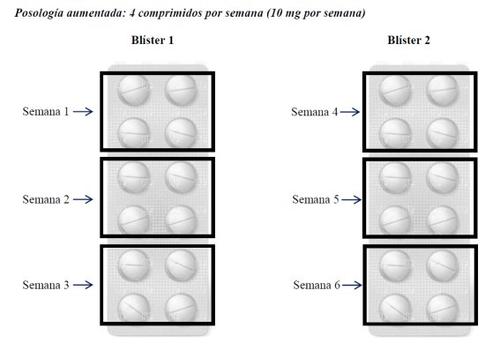
If the dose had to be increased, 1 additional tablet (2.5 mg) would be added per week, i.e., 4 tablets per week. In this case, the package of Metotrexato Weekly Wyeth with 24 tablets would cover the treatment for 6 weeks, as follows.
REACTIVE ARTHRITIS
The recommended dose is 3 tablets (7.5 mg) to 8 tablets (20 mg) once a week. Therefore, the package of Metotrexato Weekly Wyeth containing 24 tablets covers the treatment for 8 weeks at the initial dose of 3 tablets (7.5 mg), distributing the tablets as follows:
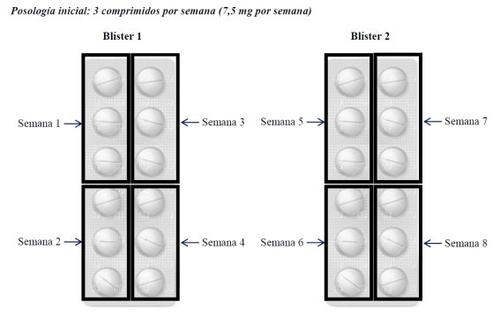
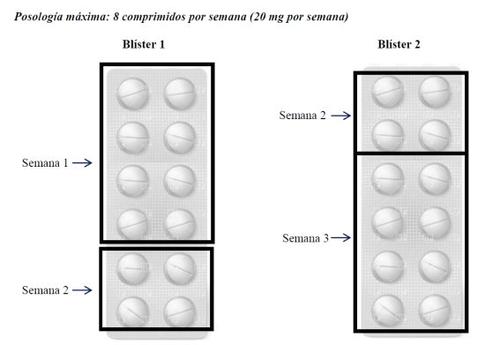
If your doctor increases your dose, they will add 1 additional tablet (2.5 mg) per week, up to a maximum of 8 tablets (20 mg) per week. Only in the case that your doctor prescribes the maximum dose, the package of Metotrexato Weekly Wyeth with 24 tablets would cover the treatment for 3 weeks, as follows.
Use in elderly patients
Elderly patients should receive a relatively low dose due to the decrease in liver and kidney function and lower folate reserves related to age.
Use in patients with kidney and liver problems
Consult your doctor, as it may be necessary to adjust the dose, and in some cases, the medication may be contraindicated.
Use in children
The doctor will calculate the necessary dose based on the child's body surface area (m2), and the dose is expressed in mg/m2.
If you take more Metotrexato Weekly Wyeth than you should
If you have taken more Metotrexato Weekly Wyeth than you should, consult your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult the Toxicological Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
An overdose of metotrexato can produce severe toxic reactions. The symptoms of overdose may include rapid formation of hematomas or bleeding, unusual weakness, sores in the mouth, nausea, vomiting, black or bloody stools, coughing up blood or vomiting with a coffee grounds appearance, and decreased urination (evacuation of urine from the bladder). See also section 4.
If you received more metotrexato than you should, it is recommended to administer folinic acid as soon as possible, as well as hydration and alkalization of the urine.
If you forget to take Metotrexato Weekly Wyeth
Do not take a double dose to make up for forgotten doses. Take the next dose when it is due.
If you interrupt treatment with Metotrexato Weekly Wyeth
Do not interrupt treatment unless your doctor tells you to. If you have any other doubts about the use of this medication, ask your doctor, pharmacist, or nurse.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medications, this medication can produce adverse effects, although not all people experience them.
If you consider that any of the adverse effects you suffer from is serious or if you notice any adverse effect not mentioned in this prospectus, inform your doctor or pharmacist.
Tell your doctor immediatelyif you experience wheezing, difficulty breathing, swelling of the eyelids, face, or lips, skin rash, or itching (especially if it affects your whole body).
Tell your doctor immediately if you notice any of the following adverse effects:
- breathing problems (symptoms may be general feeling of discomfort, dry and irritating cough, shortness of breath, difficulty breathing, chest pain, or fever)
- blood when coughing or spitting
- severe scaling or blisters on the skin
- bleeding (including blood in vomit) or unusual bruising or nosebleeds
- nausea, vomiting, abdominal discomfort, or severe diarrhea
- sores in the mouth
- black or tarry stools
- blood in urine or stools
- small red spots on the skin
- fever, sore throat, flu-like symptoms
- yellowing of the skin (jaundice) or darkening of the urine
- pain or difficulty urinating
- thirst and/or frequent need to urinate
- seizures (convulsions)
- loss of consciousness
- restricted or blurred vision
- extreme fatigue
*has been reported with metotrexato when used in patients with underlying rheumatological disease.
The following adverse effects have also been reported:
Very common(may affect more than 1 in 10 people):
- loss of appetite, nausea (vomiting), vomiting, abdominal pain, digestive problems, inflammation, and ulcers in the mouth and throat
- blood tests indicating an increase in liver enzymes.
Common(may affect up to 1 in 10 people):
- infections
- decreased formation of red blood cells, white blood cells, or platelets (leukopenia, anemia, thrombocytopenia)
- headache, fatigue, dizziness
- inflammation of the lungs (pneumonia) with dry cough, difficulty breathing, and fever
- diarrhea
- skin rash, redness of the skin, and itching.
Uncommon(may affect up to 1 in 100 people):
- lymphoma (lump in the neck, groin, or armpits, accompanied by back pain, weight loss, or night sweats)
- severe allergic reactions
- diabetes
- depression
- dizziness, confusion, seizures
- lung damage
- ulcers and bleeding in the digestive tract
- liver disease, decrease in blood proteins
- hives, reactions similar to sunburn due to increased skin sensitivity to sunlight
- brown skin discoloration, hair loss, increased number of rheumatoid nodules, shingles, painful psoriasis, slow wound healing
- joint or muscle pain, osteoporosis (decrease in bone hardness)
- kidney disease, inflammation or ulcers of the bladder (possibly also with blood in the urine), pain when urinating
- inflammation and ulcers in the vagina.
Rare(may affect up to 1 in 1,000 people):
- blood disorder characterized by the presence of very large red blood cells (megaloblastic anemia)
- mood changes
- weakness in movements, sometimes limited to the right or left side of the body
- significant visual disturbances
- inflammation of the sac surrounding the heart, accumulation of fluid in the sac surrounding the heart
- low blood pressure, blood clots
- tonsillitis, respiratory arrest, asthma
- inflammation of the pancreas, inflammation of the digestive tract, blood in the stool, inflammation of the gums, digestive problems
- acute hepatitis (liver inflammation)
- change in nail color, acne, red or purple spots due to bleeding in blood vessels
- worsening of psoriasis during treatment with UV radiation
- skin lesions similar to sunburn or radiation-induced dermatitis
- bone fractures
- kidney failure, decreased or absent production of urine, abnormal levels of electrolytes in the blood
- defective sperm formation, menstrual disorders.
Very rare(may affect up to 1 in 10,000 people):
- systemic viral, fungal, or bacterial infections
- severe bone marrow disorder (anemia), inflammation of the glands
- lymphoproliferative disorders (excessive growth of white blood cells)
- insomnia
- pain, muscle weakness, changes in taste (metallic taste), inflammation of the membrane covering the brain with paralysis or vomiting, sensation of numbness or tingling/sensitivity to stimuli less than normal
- alteration of movement of the muscles used for speech, difficulty speaking, language impairment, feeling of sleepiness or fatigue, feeling of confusion, abnormal sensations in the head, brain inflammation, ringing in the ears
- redness of the eyes, damage to the retina of the eye
- fluid accumulation in the lungs, lung infections
- vomiting blood, severe complications in the digestive tract
- liver failure
- infections of the nails, nail detachment, boils, dilation of small blood vessels, damage to blood vessels in the skin, allergic inflammation of blood vessels
- proteins in the urine
- decreased libido, erection problems, vaginal discharge, infertility, breast enlargement in men (gynecomastia)
- fever.
Frequency not known(cannot be estimated from the available data):
- pathological changes in the white matter of the brain (leukoencephalopathy)
- bleeding
- pulmonary hemorrhage
- redness and peeling of the skin
- lesion in the jawbone (secondary to an excessive increase in white blood cells)
- swelling
*has been reported with metotrexato when used in patients with underlying rheumatological disease.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Metotrexato Weekly Wyeth
Keep this medication out of sight and reach of children.
Do not store at a temperature above 25°C.
Do not use this medication after the expiration date that appears on the package after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away in drains or trash. Deposit the packages and medications you no longer need in the SIGRE point of the pharmacy. If in doubt, ask your pharmacist how to dispose of the packages and medications you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Metotrexato Weekly Wyeth
- The active ingredient is metotrexato.
- The other components are: cornstarch, lactose monohydrate, magnesium stearate, and sodium hydroxide.
Appearance of the Product and Package Contents
Metotrexato Weekly Wyeth 2.5 mg tablets are presented in PVC/aluminum blisters containing 24 tablets for oral administration.
Marketing Authorization Holder
WYETH FARMA, S.A.
Ctra. Burgos, Km. 23
San Sebastián de los Reyes
28700 – Madrid
Spain
Manufacturer
Excella GmbH & Co. KG
Nürnberger Strasse 12,
90537 Feucht,
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Pfizer, S.L.
Avda. de Europa, 20-B
Parque Empresarial La Moraleja
28108 Alcobendas (Madrid)
Spain
Date of the last revision of this prospectus:March 2025.
Detailed and updated information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price1.9 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to METOTREXATE WYETH 2.5 mg TABLETSDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Online doctors for METOTREXATE WYETH 2.5 mg TABLETS
Discuss questions about METOTREXATE WYETH 2.5 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






