
METOTREXATE CIPLA 2.5 mg TABLETS

How to use METOTREXATE CIPLA 2.5 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Methotrexate Weekly Cipla 2.5 mg Tablets EFG are and what they are used for
- What you need to know before you take Methotrexate Weekly Cipla 2.5 mg Tablets EFG
- How to Take Metotrexato Semanal Cipla Tablets
- Possible Adverse Effects
- Storage of Metotrexato Semanal Cipla 2.5 mg Tablets EFG
- Package Contents and Additional Information
- Composition of Metotrexato Cipla Tablets
Introduction
Package Leaflet: Information for the User
Methotrexate Weekly Cipla 2.5 mg Tablets EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What Methotrexate Weekly Cipla 2.5 mg Tablets are and what they are used for
- What you need to know before you take Methotrexate Weekly Cipla 2.5 mg Tablets
- How to take Methotrexate Weekly Cipla 2.5 mg Tablets
- Possible side effects
- Storing Methotrexate Weekly Cipla 2.5 mg Tablets
- Package contents and further information
1. What Methotrexate Weekly Cipla 2.5 mg Tablets EFG are and what they are used for
Methotrexate Cipla tablets contain the active substance methotrexate. Methotrexate is an antimetabolite and immunosuppressant (a medicine that affects the production of body cells and reduces the activity of the immune system).
Methotrexate is indicated for the treatment of:
- active rheumatoid arthritis in adult patients
- severe, disabling psoriasis that has not responded adequately to other treatments, such as phototherapy, UVA, and retinoids.
- severe psoriatic arthritis in adult patients.
Your doctor will tell you how Methotrexate Cipla tablets can help you in your particular situation.
2. What you need to know before you take Methotrexate Weekly Cipla 2.5 mg Tablets EFG
Do not take Methotrexate Weekly Cipla tablets
- if you are allergic to methotrexate or any of the other ingredients of this medicine (listed in section 6).
- if you are pregnant or breastfeeding (see section "Pregnancy, breastfeeding and fertility").
- if you have severe liver function disorders (your doctor will decide the severity of your disease).
- if you have severe kidney function disorders (your doctor will decide the severity of your disease).
- if you have or have had bone marrow-related diseases or severe blood disorders.
- if you have a high alcohol consumption.
- if you have any immune system disease.
- if you have severe, acute, or existing infections, such as tuberculosis or HIV.
- if you have ulcers or inflammation in the mouth.
- if you have active gastrointestinal ulcers (e.g., peptic ulcer or ulcerative colitis)
Vaccination with live vaccines should not be carried out while being treated with methotrexate
Warnings and precautions
Important dose warning for Methotrexate Weekly Cipla 2.5 mg Tablets Take Methotrexate 2.5 mg tablets only once a weekfor the treatment of rheumatic disease or psoriasis. Overuse of Methotrexate Weekly Cipla 2.5 mg tablets (methotrexate) can be fatal. Read section 3 of this leaflet very carefully. If you have any doubts, consult your doctor or pharmacist before taking this medicine |
Consult your doctor, pharmacist, or nurse before starting to take Methotrexate Weekly Cipla tablets if you have or have had any of the following conditions:
- if you have or have had any liver or kidney disease.
- if you are taking any other medicine or vitamin product (see section "Using Methotrexate Weekly Cipla tablets with other medicines").
- if you have stomach or intestinal ulcers (peptic ulcer or ulcerative colitis).
- if you have a poor general condition.
- if you have been recently vaccinated or are planning to be vaccinated.
- if you have any prolonged and inactive infection (e.g., tuberculosis, hepatitis B or C, herpes zoster virus infection).
- if you have diabetes mellitus and are being treated with insulin.
- if you have lung function problems.
- if you are severely overweight.
- if you have excess fluid in the abdomen or in the cavity between the lungs and the chest walls (ascites, pleural effusions)
- if you are dehydrated or suffer from any disease that causes dehydration (e.g., dehydration caused by vomiting, diarrhea, or inflammation of the mouth or lips).
Acute pulmonary hemorrhage has been reported with methotrexate in patients with underlying rheumatological disease. If you observe blood when coughing or spitting, you should contact your doctor immediately.
If you have skin problems after radiation therapy (radiation-induced dermatitis) or sunburn, as these conditions may recur when taking methotrexate.
Methotrexate may make your skin more sensitive to sunlight. Avoid intense sunlight and do not use sunbeds or UV lamps without medical advice. To protect your skin from intense sunlight, wear suitable clothing or use a sunscreen with a high protection factor.
Diarrhea can be a possible side effect of methotrexate and requires interruption of treatment. If you suffer from diarrhea, please talk to your doctor.
Special precautions for treatment with Methotrexate Weekly Cipla
Methotrexate temporarily affects the production of sperm and eggs, which is reversible in most cases. Methotrexate can cause abortions and severe birth defects. If you are a woman, you must avoid becoming pregnant while using methotrexate and for at least six months after stopping treatment. If you are a man, you must avoid fathering a child if you are being given methotrexate at that time and for at least 3 months after stopping treatment. See also section "Pregnancy, breastfeeding and fertility".
Skin changes caused by psoriasis may worsen during treatment with methotrexate if you are exposed to ultraviolet radiation.
Brain disease (encephalopathy/leukoencephalopathy) has been reported as a side effect in patients receiving methotrexate for cancer treatment; it cannot be excluded that this may also occur when taking methotrexate tablets for the treatment of rheumatoid arthritis or psoriasis.
If you, your partner, or your caregiver notice the appearance or worsening of neurological symptoms, such as general muscle weakness, vision disturbances, changes in thinking, memory, and orientation that cause confusion and changes in personality, contact your doctor immediately because these may be symptoms of a rare and severe brain infection called progressive multifocal leukoencephalopathy (PML).
Recommended precautions and complementary tests
Even if methotrexate is used at low doses, serious side effects may occur. To detect them in time, your doctor should perform control tests and analytical tests.
Before starting treatment:
Before starting treatment, your blood will be tested to see if you have enough blood cells. Your blood will also be tested to check your liver function and to see if you have hepatitis. Additionally, your serum albumin (a protein in the blood), hepatitis status (liver infection), and kidney function will be checked. Your doctor may also decide to perform other liver tests, some of which may involve imaging of your liver and others may require a small tissue sample from your liver for closer examination. Your doctor may also check if you have tuberculosis and take a chest X-ray or perform a respiratory function test.
During treatment:
Your doctor may perform the following tests:
- examine the oral cavity and pharynx to detect changes in the mucosa, such as inflammation or ulceration;
- blood tests/complete blood count with blood cell count and measurement of serum methotrexate levels;
- blood tests to monitor liver function;
- imaging tests to monitor liver condition;
- small tissue sample taken from the liver for closer examination;
- blood tests to monitor kidney function;
- monitoring of the respiratory tract and, if necessary, pulmonary function test.
It is very important that you attend these scheduled tests.
If the results of any of these tests are remarkable, your doctor will adjust your treatment accordingly.
Elderly patients
Elderly patients treated with methotrexate should be closely monitored by a doctor so that possible side effects can be detected as soon as possible.
Age-related deterioration of liver and kidney function, as well as low body reserves of folic acid in old age, require a relatively low dose of methotrexate.
Children and adolescents
Methotrexate tablets are not indicated for children or adolescents for the treatment of rheumatoid arthritis and psoriasis.
Using Methotrexate Weekly Cipla tablets with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Taking other medicines at the same time as this medicine may affect its efficacy and safety.
Remember to tell your doctor that you are being treated with methotrexate tablets if another medicine is prescribed for you while you are still being treated. It is especially important that you tell your doctor if you are using:
- metamizole (synonyms novaminsulfon and dipyrone) (medicine for intense pain and/or fever);
- certain antibiotics (chloramphenicol, penicillins, glycopeptides, sulfonamides, ciprofloxacin, cefalotin, trimethoprim/sulfamethoxazole, and tetracyclines);
- diuretics (triamterene);
- medicines to lower blood sugar, such as metformin;
- anticonvulsant medicines, such as phenytoin or levetiracetam (medicine for the treatment of epilepsy);
- a medicine that sequesters bile acids and may be used to lower cholesterol levels (cholestyramine).
- probenecid (medicine for the treatment of gout);
- folic acid (vitamin preparation);
- omeprazole or pantoprazole (medicines for stomach acidity);
- medicines that produce liver and kidney toxicity [such as sulfasalazine and leflunomide (medicines for the treatment of rheumatic diseases) and alcohol];
- anticancer agents (e.g., doxorubicin, cisplatin, mercaptopurine);
- medicines used for pain and/or inflammation, known as non-steroidal anti-inflammatory drugs (e.g., diclofenac and ibuprofen, salicylates such as acetylsalicylic acid (aspirin), and pyrazoles such as metamizole);
- other medicines for rheumatoid arthritis or psoriasis, such as azathioprine, leflunomide, sulfasalazine (a medicine that, in addition to rheumatoid arthritis and psoriasis, is also used to treat ulcerative colitis), phenylbutazone, or aminopyrine;
- theophylline (medicine for the treatment of respiratory diseases);
- cyclosporin (an immune response suppressor or preventive agent).
- Barbiturates (sleep injection)
- Tranquilizers
- Oral contraceptives
- Retinoids (used to treat psoriasis and other skin disorders)
- Pyrimethamine (used to prevent or treat malaria)
- Any type of vaccination with live vaccines (should be avoided), such as measles, mumps, flu virus, or yellow fever.
Tell your doctor about the use of Methotrexate Weekly Cipla tablets during your next visits.
Using Methotrexate Weekly Cipla tablets with food, drinks, and alcohol
Alcohol consumption should be avoided, as well as excessive consumption of coffee, caffeinated soft drinks, and black tea, as they may increase the side effects or interfere with the efficacy of methotrexate while being treated with it. Additionally, you should ensure that you drink a sufficient amount of fluids during treatment with methotrexate, as it may increase the risk of dehydration (reduction of body water) and thus increase the toxicity of methotrexate.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Do not use Methotrexate Weekly Cipla during pregnancy or if you are trying to become pregnant. Methotrexate can cause birth defects, harm the fetus, or cause abortions. It is associated with malformations of the skull, face, heart, and blood vessels, brain, and limbs. Therefore, it is very important that methotrexate is not administered to pregnant patients or those planning to become pregnant. In women of childbearing age, any possibility of pregnancy should be excluded with appropriate measures, such as a pregnancy test before starting treatment. You must avoid becoming pregnant while taking methotrexate and for at least 6 months after stopping treatment, using reliable contraceptive methods during this time (see also section "Warnings and precautions").
If you become pregnant during treatment or think you may be pregnant, consult your doctor as soon as possible. You will be offered information about the risk of harmful effects on the child during treatment.
If you wish to become pregnant, consult your doctor, who may refer you to a specialist for information before the planned start of treatment.
Breastfeeding
Do not breastfeed during treatment with methotrexate, as methotrexate has been detected in breast milk. If your doctor considers it absolutely necessary to take the medicine, stop breastfeeding your baby.
Fertility
Male fertility
Available data do not indicate an increased risk of malformations or abortions if the father takes a methotrexate dose of less than 30 mg/week. However, this risk cannot be completely ruled out. Methotrexate may be genotoxic, which means it can cause genetic mutations. Methotrexate may affect sperm production and cause birth defects. Therefore, you must avoid fathering a child or donating sperm while taking methotrexate and for at least 3 months after stopping treatment.
Consult your doctor or pharmacist before starting treatment.
Driving and using machines
During treatment with methotrexate, you may feel tired and dizzy. Do not drive or use machines if you have these symptoms.
Methotrexate Weekly Cipla tablets contain lactose and sodium
If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
This medicine contains less than 1 mmol of sodium (23 mg) per tablet; this is essentially "sodium-free".
3. How to Take Metotrexato Semanal Cipla Tablets
Follow the administration instructions of this medication exactly as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
- Take metotrexato tablets once a week.
- Patient with rheumatoid arthritis, psoriatic arthritis, or psoriasis should take their tablets orally once a weekon the same day each week.
- Do not take the tablets more frequently than indicated by your doctor.
- Daily administration may produce severe toxic effects, including death.
- Take the tablets with a glass of water while sitting or standing.
The recommended dose is
Rheumatoid Arthritis
The recommended dose is 7.5 to 15 mg orally, once a week.
Psoriasis
The recommended dose is 7.5 to 15 mg orally, once a week.
Take metotrexato only once a week
Information on taking tablets for rheumatic or skin diseases in adults.
Each package of metotrexato 2.5 mg tablets contains 24 tablets. Each tablet contains a dose of 2.5 mg of metotrexato. The following graphic schemes show the recommended amount of tablets to be taken for each of the inflammatory indications described above. These diagrams are examples, and your doctor may change the dose if required. It is very important that you take the correct number of tablets prescribed by your doctor. Also, remember that taking the tablets indicated by your doctor must be done 1 time per week. This medication SHOULD NOT BE TAKEN EVERY DAY for the treatment of rheumatoid arthritis and psoriasis.
Rheumatoid Arthritis and Psoriasis
The initial dose in adults is 3 tablets (7.5 mg) once a week. Therefore, the package of metotrexato containing 24 tablets covers the treatment for 8 weeks, distributing the tablets as follows:
Initial Posology: 3 tablets of 2.5 mg per week (7.5 mg per week)
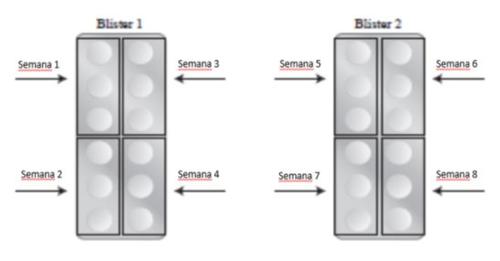
Increased Posology: 4 tablets of 2.5 mg per week (10 mg per week)
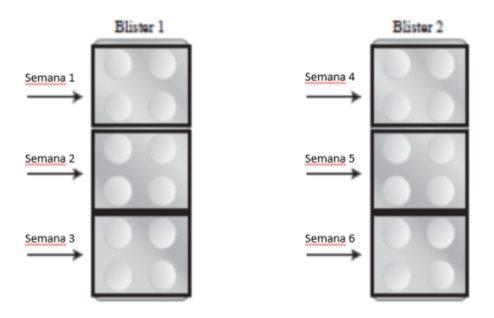
The dose should be adjusted according to your response to treatment and adverse effects.
Proper procedures should be used for the safe handling of cytotoxic agents. Anyone who must come into contact with metotrexato should wash their hands once they have administered a dose. Gloves should be used when coming into contact with metotrexato tablets. Pregnant or breastfeeding women or those planning to become pregnant should not come into contact with metotrexato.
Use in Children
Administration to children is not recommended.
If You Take More Metotrexato Cipla Tablets Than You Should
If you (or someone else) take more medication than you should, consult a doctor or go to the nearest hospital immediately. In case of overdose or accidental ingestion, consult the Toxicology Information Service, phone 91 562 04 20.
An overdose of metotrexato can cause severe toxic reactions, including death. The symptoms of overdose may include: bruising or bleeding, unusual weakness, sores in the mouth, nausea, vomiting, black stools or with blood, coughing up blood or vomiting with a coffee grounds appearance, and decreased urine production. See section 4.
Take the medication with you if you go to the doctor or hospital.
If You Forget to Take Metotrexato Cipla Tablets
If you forget to take your dose, take it as soon as you remember, provided it is within the next two days. However, if you forget to take your dose for more than two days, contact your doctor for advice. Do not take a double dose to make up for forgotten doses.
Make sure you have enough medication before traveling or vacationing.
If You Stop Treatment with Metotrexato Cipla Tablets
Do not stop treatment with metotrexato unless your doctor tells you to. If you need to stop treatment, your doctor will decide the best method for you.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medications, this medication can cause adverse effects, although not everyone will experience them.
In general, the incidence and severity of metotrexato's adverse effects are related to the dose and frequency of administration. Most adverse effects are reversible if detected early.
Severe Adverse Effects
If you experience any of the following symptoms, contact your doctor or go to the hospital immediately.
Uncommon (may affect 1 in 100 people)
- A cough that produces thick mucus, difficulty breathing, fever, or short breaths. You may have pulmonary fibrosis or pneumonia.
- Chest pain, difficulty breathing, swelling of the face, throat, or hands, feeling of dizziness or fainting. These could be signs of a severe allergic reaction.
- Severe skin reactions such as peeling and blistering in the mouth, eyes, and genitals, as well as numerous eruptions with pus, fever. You may be suffering from Stevens-Johnson syndrome or toxic epidermal necrolysis.
- Reactions similar to sunburn due to increased skin sensitivity to sunlight.
- Fever and general deterioration, or fever with local infections such as in the throat or mouth. You may have a reduced number of white blood cells (possibly due to bone marrow depression) and may decrease your resistance to infections.
- Pain or difficulty urinating.
- Kidney impairment.
Rare (may affect up to 1 in 1,000 people)
- Loss of appetite, nausea, skin itching, yellowing of the skin or eyes, fever, swelling or abdominal distension. You may be suffering from liver damage or inflammation.
- Vomiting blood, black stools like tar, and stomach pain. You may be suffering from a stomach hemorrhage or ulcer.
- Pain like cramps, acute pain, or swelling in the leg, redness, difficulty breathing, chest pain, or sudden collapse. You may have a blood clot.
- Reduced or lost urine production.
- Fever, chills, and shivering, rapid heartbeat, rapid breathing, confusion, or dizziness. You may present with sepsis as a result of an infection.
Very Rare (may affect up to 1 in 10,000 people)
- Blood in the urine.
- Difficulty speaking or communicating.
- Seizures.
Unknown (frequency cannot be estimated from available data)
- Blood when coughing or spitting.
- Certain brain disorders (encephalopathy/leukoencephalopathy).
Other Adverse Effects
Very Common (may affect more than 1 in 10 people)
- inflammation of the throat or pain in the mouth or lips,
- indigestion,
- loss of appetite,
- nausea,
- vomiting,
- stomach pain,
- increase in liver enzymes
Frequent(may affect 1 in 10 people)
- infections
- diarrhea
- exhaustion
- fatigue
- headache
- dizziness
- redness or large red patches on the skin
- hair loss
- drowsiness
- rash
- mouth ulcers
Uncommon(may affect 1 in 100 people)
- reduction in blood clotting
- change in blood cell count
- anemia
- skin itching
- Inflammation in the lymph nodes that can be a sign of cancer of the lymphatic system (lymphoma)
- Vaginal inflammation and ulcers
- vertigo
- fatty liver
- decrease in serum albumin levels
- herpes-like rashes on the skin
- increased skin pigmentation
- osteoporosis
- pain in muscles and joints
- appearance of local lumps in tissues
- bladder ulcers
- chills
Rare(may affect 1 in 1,000 people)
- depression
- confusion
- hemiparesis (weakness that affects only one side of the body)
- diabetes
- low blood pressure (hypotension)
- shortness of breath
- gum inflammation
- throat pain
- acne
- skin lightening
- pruritic skin rash
- burning sensation in psoriatic skin lesions
- skin ulcers
- herpes or painful rashes
- menstrual disorders
- impotence
- reduced sexual desire
- Blood disorder characterized by the appearance of very large red blood cells (megaloblastic anemia)
- Mood swings
- Inflammation of the sac surrounding the heart, accumulation of fluid in the cardiac sac
- Inflammation of the small intestine
- Blood in stools
- Nail detachment
- Dark areas on the nails
- Red or purple spots due to bleeding from blood vessels
- Allergic inflammation of blood vessels
- Skin lesions similar to sunburn or radiation dermatitis
- Stress fractures
- Abnormal levels of electrolytes in the blood
- Physical weakness
- Fever
- Slow wound healing
- Respiratory arrest
Very Rare(may affect 1 in 10,000 people)
- immune deficiency (hypogammaglobulinemia)
- irritation
- drowsiness, fatigue (lethargy)
- vision changes
- redness and irritation of the thin membrane that covers the eye (conjunctivitis)
- fluids or swelling in the sac that surrounds the heart or lungs
- pulmonary infection
- dry cough
- vomiting blood
- boils
- hematomas or capillaries on the skin surface
- fertility problems
- reduced sperm count
- infertility
- vaginal bleeding or discharge
- enlargement of male breast tissue
- lymphoproliferative disorders (excessive increase in white blood cells)
- Severe bone marrow disorders
- Increased susceptibility to infections
- Insomnia
- Psychosis
- Low levels of antibodies
- Mild temporary disorder of intellectual function ("mental fog")
- Having strange sensations in the head
- Brain inflammation
- Ringing in the ears
- Pain
- Muscle weakness
- Numbness
- Changes in taste (metallic taste)
- Inflammation of the membranes of the brain
- Paralysis
- Inflammation of the membranes that cover the lungs
- Liver failure
- Inflammation of blood vessels
- Chronic obstructive pulmonary disease
- Inflammation of sweat glands
- Nail infections
- Sensation of numbness or tingling/sensitivity to stimuli less than normal
Unknown(frequency cannot be estimated from available data)
- abnormally low blood cell count
- sepsis with fatal outcome
- fetal death
- fetal damage
- increased risk of toxicity during radiation therapy
- increase in white blood cell count and inflammation of lung tissue
- may worsen red and scaly plaques associated with psoriasis when exposed to ultraviolet light, such as with the sun, along with taking metotrexato
- Pulmonary hemorrhage
- Jawbone injury (secondary to excessive increase in white blood cells)
- Reactivation of inactive chronic infections
- Vision problems
- Damage to the retina of the eye
- Colon enlargement due to inflammation/infection
- Pancreatitis
- Pathological change in the white matter of the brain (leukoencephalopathy)
- Nasal bleeding
- Asthma
- Presence of proteins in the urine
- Redness and peeling of the skin
- Swelling
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Metotrexato Semanal Cipla 2.5 mg Tablets EFG
Keep this medication out of sight and reach of children.
This medication does not require any special storage temperature.
Blister: Store the blister in the outer packaging to protect it from light.
HDPE Bottle: Store in the original packaging to protect it from light.
Do not use this medication after the expiration date that appears on the packaging after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away in drains or trash. Deposit the packaging and medications you no longer need in the SIGRE point of the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Metotrexato Cipla Tablets
- The active ingredient is metotrexato.
- Metotrexato Cipla 2.5 mg tablets: each tablet contains 2.5 mg of metotrexato.
- The other components are: anhydrous calcium hydrogen phosphate, lactose monohydrate, sodium glycolate starch, microcrystalline cellulose, talc, and magnesium stearate.
Appearance of the Product and Package Contents
The Metotrexato Cipla 2.5 mg tablets are yellow, circular, biconvex, and smooth on both sides with dimensions of 4.50 mm ± 0.2 mm.
The Metotrexato 2.5 mg tablets are presented in HDPE bottles containing 25 or 100 tablets and in blister packaging of 10, 24, 25, 28, 30, 50, or 100 tablets.
Only some package sizes may be marketed.
Marketing Authorization Holder
Cipla Europe NV
De Keyserlei 58-60,
Box 19, 2018 Antwerp
Belgium.
Manufacturer
Cipla Europe NV
De Keyserlei 58-60,
Box 19, 2018 Antwerp
Belgium.
S&D Pharma CZ
spol. s.r.o, Theodor 28
Pchery (Pharmos a.s. facility), 27308
Czech Republic.
Local Representative
Cipla Europe NV branch in Spain
C/ Guzmán el Bueno, 133 Edif Britannia
28003 Madrid
Spain
This medication is authorized in the Member States of the European Economic Area with the following names:
Croatia | Metotreksat Cipla 2,5 mg tablets |
Spain | Metotrexato Semanal Cipla 2,5 mg tablets EFG |
Norway | Methotrexate Cipla |
Date of the Last Revision of this Leaflet: October 2024
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Patient Card
THIS PATIENT CARD IS INTENDED ONLY FOR PATIENTS WHO USE MEDICATIONS CONTAINING METOTREXATO FOR ARTHRITIS AND PSORIASIS.
IF YOU USE METOTREXATO FOR ANY OF THE DISEASES MENTIONED ABOVE, YOU SHOULD TAKE METOTREXATO ONLY ONCE A WEEK.
Write here in full the day of the week on which it is administered: ______________
Do not take a dose larger than the one prescribed for you.
Overdose can cause severe adverse effects and can be fatal. The symptoms of overdose are, for example: sore throat, fever, mouth ulcers, diarrhea, vomiting, skin rashes, bleeding, or unusual weakness. If you think you have exceeded the prescribed dose, go to a doctor immediately.
Always show this card to healthcare professionals who are not familiar with your treatment with metotrexato to alert them to its weekly use (for example, when admitted to the hospital, changing caregiver).
For more information, read the leaflet included in the packaging.
Information on risk prevention authorized by the Spanish Agency for Medicines and Health Products (AEMPS). April 2020.
Available on the AEMPS website www.aemps.gob.es
- Country of registration
- Average pharmacy price1.9 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to METOTREXATE CIPLA 2.5 mg TABLETSDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Online doctors for METOTREXATE CIPLA 2.5 mg TABLETS
Discuss questions about METOTREXATE CIPLA 2.5 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






