
MERIOFERT KIT 900 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use MERIOFERT KIT 900 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Meriofert Kit 900 UIpowder and solvent for solution for injection
Menotropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
- In this leaflet, Meriofert Kit 900 UI powder and solvent for solution for injection is referred to as Meriofert Kit.
Contents of the pack
- What is Meriofert Kit and what is it used for
- What you need to know before you use Meriofert Kit
- How to use Meriofert Kit
- Possible side effects
- Storing Meriofert Kit
- Contents of the pack and other information
1. What is Meriofert Kit and what is it used for
- Meriofert Kit is used to stimulate ovulation in women who do not ovulate and have not responded to other treatment (clomifene citrate).
responded to other treatment (clomifene citrate).
- Meriofert Kit is used to induce the development of multiple follicles (and therefore multiple eggs) in women undergoing fertility treatment.
Meriofert Kit is a highly purified human menopausal gonadotropin, which belongs to a group of medicines called gonadotropins.
Each multidose vial contains lyophilized powder with 900 IU of human follicle-stimulating hormone (FSH) activity and 900 IU of human luteinizing hormone (LH) activity.
The human menopausal gonadotropin (HMG) is extracted from the urine of postmenopausal women. Human chorionic gonadotropin (hCG) is added, extracted from the urine of pregnant women, to contribute to the total LH activity.
This medicine should be used under the supervision of a doctor.
2. What you need to know before you use Meriofert Kit
Before starting treatment, your fertility and that of your partner will be assessed.
Do not use Meriofert Kit
- If you have enlarged ovaries or cysts of unknown origin (polycystic ovarian disease)
- If you have unexplained bleeding
- If you have ovarian, uterine, or breast cancer
- If you have an abnormal swelling (tumor) of the pituitary gland or hypothalamus (brain)
- If you are allergic to menotropin or any of the other ingredients of this medicine (listed in section 6).
Do not use this medicine if you have premature menopause, malformation of the genital organs, or certain uterine tumors that would prevent a normal pregnancy.
Warnings and precautions
Although allergic reactions to Meriofert Kit have not been described, you should inform your doctor if you have any allergic reactions to similar medicines.
This treatment increases the risk of a disease called ovarian hyperstimulation syndrome (OHSS)(see Possible side effects). If ovarian hyperstimulation occurs, treatment will be stopped and pregnancy should be avoided. The first signs of ovarian hyperstimulation are pain in the lower abdomen, nausea (discomfort), vomiting, and weight gain. If these symptoms occur, you should see a doctor as soon as possible. In severe cases, the ovaries may become enlarged and fluid may accumulate in the abdomen or chest.
The medicine used to trigger the final release of mature eggs (which contains hCG) may increase the likelihood of OHSS. Therefore, it is not recommended to use hCG in cases where OHSS is occurring, and you should not have sexual intercourse for at least 4 days, even if you use a barrier contraceptive method.
It is worth noting that women with fertility problems have a higher rate of miscarriages than the normal population.
The frequency of pregnancies and multiple births in patients undergoing ovulation stimulation treatment is higher than in women who conceive naturally. However, this risk can be minimized if the recommended dose is used.
The risk of ectopic pregnancy (pregnancy outside the uterus) is slightly higher in women with damaged fallopian tubes.
Multiple pregnancies and the characteristics of parents undergoing fertility treatment (e.g., the mother's age or semen characteristics) may be associated with an increased risk of birth defects.
Treatment with Meriofert Kit, like pregnancy itself, may increase the likelihood of suffering a blood clot. A blood clot is the formation of a blood clot in a blood vessel, most often in the veins of the legs or lungs.
Discuss this with your doctor before starting treatment, especially:
- If you already know you have a higher risk of suffering a blood clot.
- If you or a close family member has ever suffered a blood clot.
- If you are severely overweight.
Pediatric population
This medicine is not indicated for pediatric use.
Using Meriofert Kit with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
Meriofert Kit should not be used if you are pregnant or breastfeeding.
Driving and using machines
Meriofert Kit has no or negligible influence on the ability to drive and use machines.
Meriofert Kit contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially “sodium-free”.
3. How to use Meriofert Kit
Dose and duration of treatment
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If in doubt, consult your doctor again.
Women who do not ovulate and have irregular periods or absence of menstruation:
As a general rule, the first injection of a vial of 75 IU of menotropin is administered during the first week of the cycle after spontaneous or induced menstruation.
Subsequently, the prescribed dose of this medicine is injected every day, and treatment is continued until one or more mature follicles have formed in the ovary. Your doctor will adjust the dose of this medicine based on the ovarian response, which is determined by clinical examinations.
Once a follicle reaches the necessary stage of development, treatment with this medicine will be stopped, and ovulation will be triggered with another hormone (human chorionic gonadotropin, hCG).
Ovulation usually occurs within 32 to 48 hours.
In this phase of treatment, there is a possibility of fertilization. You will be advised to have sexual intercourse every day starting from the day before hCG administration. If pregnancy is not achieved despite ovulation, treatment may be repeated.
Women undergoing ovarian stimulation for multiple follicular development before in vitro fertilization or other assisted reproduction techniques
The purpose of this method is to achieve simultaneous multiple follicular development. Treatment will begin on the second or third day of the cycle with injections of 150 to 300 IU of Meriofert Kit. Your doctor may choose to administer higher doses if necessary. The dose of this medicine injected is higher than that used for natural fertilization. Your doctor will adjust the continuation of treatment on an individual basis.
Once a sufficient number of follicles have developed, treatment with this medicine will be stopped, and ovulation will be triggered by injection of another hormone (human chorionic gonadotropin, hCG).
How to administer Meriofert Kit:
This medicine is administered by subcutaneous injection.
Vials should only be reconstituted once, and each single injection should be administered as soon as the required dose has been withdrawn.
After advising and instructing you properly, your doctor may ask you to administer the Meriofert Kit injection yourself.
Before the first injection, your doctor should:
- Allow you to practice self-administration of a subcutaneous injection
- Indicate the areas where the injection can be administered
- Teach you how to prepare the injectable solution
- Explain how to prepare the correct dose for injection.
Before administering the Meriofert Kit injection, read the following instructions carefully.
Since this vial contains medication for several days of treatment, you must ensure that you only withdraw the amount of medication prescribed by your doctor. Your doctor has prescribed a dose of Meriofert Kit in IU (units). To obtain the correct dose, you must use one of the 12 graduated syringes for administration in FSH/LH units provided in the package.
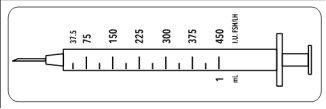
These disposable syringes are intended for single use and should be discarded after administration, according to local regulations, in an appropriate container.
How to prepare and inject 1 vial of Meriofert Kit:
The injection solution containing 900 IU of menotropin should be prepared just before the first dose is to be administered. To do this, add the reconstitution solvent from the pre-filled syringe (included in the package) to the vial containing the powder.
Prepare a clean surface and wash your hands with soap and warm water. It is essential that your hands and the items you use are as clean as possible.
Place the following items on the surface:
- the vial of Meriofert Kit powder
- the pre-filled syringe with reconstitution solvent
- the needle for reconstitution
- a disposable subcutaneous injection syringe with pre-attached needle graduated in FSH/LH units
- a cotton swab with alcohol
- cotton and disinfectant solution (not included in the package)
REMEMBER:
- Disinfect the rubber stopper of the vial containing the reconstituted solution with a cotton swab and disinfectant (i.e., alcoholic solution) and let it dry before reconstitution and each administration.
- Do not remove the support ring (white piece) from the pre-filled syringe, as it prevents accidental withdrawal of the piston and improves handling of the syringe during injection.
Reconstitution of the injectable solution
Preparation of the pre-filled syringe:
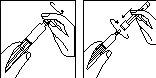
1.
- Remove the cap from the pre-filled syringe with solvent; attach the reconstitution needle with the protective cap still on the syringe.
- Place the syringe carefully on a clean surface.
Preparation of the vial:
2.
- Remove the colored plastic flip-off cap from the vial by gently pushing it upwards with your thumb.
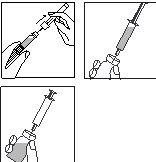 Clean the rubber stopper with a cotton swab and disinfectant and let it dry.
Clean the rubber stopper with a cotton swab and disinfectant and let it dry.
3.
- Take the syringe, remove the protective cap from the needle, and push the needle through the center of the rubber stopper of the vial.
- Push the plunger firmly to empty all the solvent into the powder.
- When adding the solvent, a slight overpressure is created in the vial. Therefore, release the syringe plunger so that it rises by itself for about 10 seconds. This will eliminate the overpressure in the vial.
DO NOT SHAKE the reconstituted solution, gently swirl it until a clear solution is obtained. The medicine usually dissolves immediately.
Check that the reconstituted solution is clear.
Before injection:
- Check that the reconstituted solution is transparent, colorless, and free of particles. DO NOT USE if the solution contains particles, is cloudy, or is not colorless.
- Clean the rubber stopper with a cotton swab and disinfectant.
Preparation of the injection:
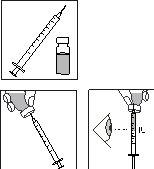
4.
- Take a disposable syringe with pre-attached needle, remove the protective cap from the needle, and insert the needle vertically into the center of the vial stopper.
- Push the plunger until it is fully pressed.
- Invert the vial. Make sure the needle is below the surface of the medicine and withdraw the prescribed dose of medicine into the administration syringe.
- Remove the needle from the vial. Hold the syringe with the needle pointing upwards and gently tap the side of the syringe to force any air bubbles upwards.
- Slowly push the plunger until a drop of liquid appears at the tip of the needle.
REMEMBER: since the vial contains medication for several days of treatment, you must ensure that you only withdraw the amount of medication prescribed by your doctor.
Administration of the injection
Injection site:
 Your doctor or nurse will have already explained where on your body the medicine should be injected. The usual places are the thigh or the lower abdominal wall below the navel.
Your doctor or nurse will have already explained where on your body the medicine should be injected. The usual places are the thigh or the lower abdominal wall below the navel.
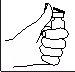
- Clean the injection site with a cotton swab with alcohol.
- Pinch and firmly press the skin. With the other hand, insert the needle with a quick, firm motion, forming a 45° or 90° angle.
Injection of the solution:
- Inject the syringe under the skin as indicated. Do not inject it directly into a vein. Slowly push the plunger without interruption, so that the solution is injected correctly and the skin tissues are not damaged.
Take the time you need to inject the prescribed volume of solution.
Quickly remove the needle and press the injection site with a cotton swab with disinfectant. Gently massage the area (while maintaining pressure), this helps to disperse the medicine and alleviate any discomfort.
Subsequent injections:
Repeat from step 4 onwards for subsequent injections with the reconstituted Meriofert Kit solution.
If you use more Meriofert Kit than you should
The effects of overdose of this medicine are unknown; however, it is likely that ovarian hyperstimulation syndrome (see Possible side effects) would occur. If you use more medicine than you should, consult your doctor or nurse.
If you forget to use Meriofert Kit
Use it at the next scheduled time. Do not take a double dose to make up for forgotten doses.
If you stop using Meriofert Kit
Do not stop treatment on your own initiative. Always consult your doctor before stopping this medicine.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effect is important and will require immediate action if you experience it. You must stop taking this medicine and see a doctor immediately if you experience the following:
Common (may affect up to 1 in 10 people):
- Ovarian hyperstimulation syndrome (symptoms include the formation of ovarian cysts or the enlargement of existing cysts, pain in the lower abdomen, feeling of thirst, and nausea with occasional vomiting, passing small amounts of concentrated urine, and weight gain) (see section 2 for additional information).
The following side effects have also been reported:
Very common (may affect more than 1 in 10 people):
- Headache
- Abdominal bloating
Common (may affect up to 1 in 10 people):
- Abdominal pain or discomfort
- Pelvic pain
- Back pain
- Feeling of heaviness
- Breast tenderness
- Dizziness
- Hot flashes
- Thirst
- Feeling sick
- Fatigue
- General malaise
- Reactions at the injection site, such as pain and inflammation
Rare (may affect up to 1 in 1,000 people):
- Ovarian torsion (twisting of the ovary causing severe pain in the lower abdomen)
- Thromboembolism (formation of a blood clot in a blood vessel that breaks loose and is carried by the bloodstream to block another vessel).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Meriofert Kit
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging, vial, and prefilled syringe of solvent after CAD. If the expiry date is indicated as month/year, the expiry date is the last day of the indicated month.
Before reconstitution: Store in a refrigerator (between 2°C and 8°C).
After reconstitution, the solution can be stored for a maximum of 28 days at no more than 25°C.
Do not freeze before or after reconstitution.
Do not use this medicine if you notice that the solution is not transparent. After reconstitution, the solution should be transparent and colorless.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need at the pharmacy's SIGRE point  . In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Meriofert Kit
The active ingredient is menotropin.
Each multidose vial contains lyophilized powder with 900 IU of human follicle-stimulating hormone (FSH) activity and 900 IU of human luteinizing hormone (LH) activity.
Human menopausal gonadotropin (HMG) is extracted from the urine of postmenopausal women. Human chorionic gonadotropin (hCG) is added, extracted from the urine of pregnant women, to contribute to the total LH activity.
The other components are
Powder: lactose monohydrate, polysorbate 20, sodium dihydrogen phosphate dihydrate, phosphoric acid, and sodium hydroxide.
Solvent: metacresol and water for injectable preparations.
Appearance of Meriofert Kit and Package Contents
Powder: white lyophilized cake or powder.
Solvent: clear and colorless solution.
Meriofert Kit is presented as a powder and solvent for injectable solution.
A carton contains:
- 1 vial of Meriofert Kit powder
- 1 prefilled syringe with solvent for reconstitution
- 1 needle for reconstitution
- 12 cotton swabs with alcohol for multiple injections
- 12 disposable syringes with pre-fixed needles for multiple injections
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
IBSA FARMACEUTICI ITALIA SRL
Via Martiri di Cefalonia 2
26900 Lodi, Italy
Manufacturer
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italy
or (for the United Kingdom/Northern Ireland)
IBSA Pharma Limited
Units 4-6
Colonial Business Park
Colonial Way
Watford WD24 4PR
United Kingdom
You can request more information about this medicine by contacting the local representative of the Marketing Authorization Holder:
Instituto Bioquimico Iberico IBSA S.L.
Avenida Diagonal 605,
Planta 8, Local 1,
08028 Barcelona (Spain)
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) with the following names (concentrations and pharmaceutical forms are identical in all countries, only the trade names differ):
Austria: Meriofert PFS
Belgium: Fertinorm Kit
Bulgaria: Meriofert PFS
Cyprus: Meriofert PFS
Czech Republic: Meriofert Set
Denmark: Meriofert Set
Estonia: Meriofert Set
Finland: Meriofert Set
France: Fertistartkit
Greece: Meriofert
Hungary: Meriofert Kit
Italy: Meriofert
Latvia: Meriofert Set
Lithuania: Meriotert Set
Luxembourg: Fertinorm Kit
Norway: Meriofert Set
Poland: Mensinorm Set
Romania: Meriofert PFS
Slovakia: Meriofert Kit
Spain: Meriofert Kit
Sweden: Meriofert Set
Netherlands: Meriofert spuit
United Kingdom: Meriofert PFS
Date of the last revision of this leaflet: May 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MERIOFERT KIT 900 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, -Active substance: human menopausal gonadotrophinManufacturer: Angelini Pharma Espana S.L.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.U.Prescription required
Online doctors for MERIOFERT KIT 900 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about MERIOFERT KIT 900 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






