
LIPOFLEX PLUS EMULSION FOR INFUSION

How to use LIPOFLEX PLUS EMULSION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Lipoflex Plus Emulsion for Infusion EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Lipoflex Plus and what is it used for
- What you need to know before you use Lipoflex Plus
- How to use Lipoflex Plus
- Possible side effects
- Storage of Lipoflex Plus
- Contents of the pack and further information
1. What is Lipoflex Plus and what is it used for
Lipoflex Plus contains liquids and substances called amino acids, electrolytes, and fatty acids that are essential for the growth or recovery of the body. It also contains calories in the form of carbohydrates and fats.
This medicine is given when there is an inability to eat food normally. There are many situations where this can happen, such as during the recovery phases of surgical interventions, trauma, or burns, or when there is an inability to absorb food in the stomach and intestine.
This emulsion can be administered to adults, adolescents, and children over 2 years of age.
2. What you need to know before you use Lipoflex Plus
Do not use Lipoflex Plus
? if you are allergic to any of the active substances, egg, peanut, or soy, or to any of the other components of this medicine (listed in section 6),
? this medicine must not be administered to newborns, infants, and children under two years of age.
Also, do not use this medicine if you have any of the following disorders:
? potentially life-threatening blood circulation problems, such as those that can occur in cases of collapse or shock,
? myocardial infarction or stroke,
? severe blood coagulation disorders, risk of bleeding (severe coagulopathy, bleeding diathesis),
? blockage of blood vessels by blood clots or fat (embolism),
? severe liver failure,
? altered bile flow (intrahepatic cholestasis),
? severe kidney failure where dialysis equipment is not available,
? alterations in the body's salt composition,
? fluid deficit or excess water in your body,
? water in your lungs (pulmonary edema),
? severe heart failure,
? certain metabolic disorders, such as:
- too much fat (lipids) in the blood,
- congenital disorders of amino acid metabolism,
- abnormally high blood sugar levels that require more than 6 units of insulin per hour to be controlled,
- metabolic disorders that can occur after surgical interventions or trauma,
- coma of unknown origin,
- insufficient oxygen supply to the tissues,
- abnormally high acid levels in the blood.
Warnings and Precautions
Consult your doctor, pharmacist, or nurse before starting to use Lipoflex Plus.
Tell your doctor if:
? you have heart, liver, or kidney problems,
? you have certain types of metabolic disorders, such as diabetes, abnormal blood fat values, and disorders of body fluid and salt composition or acid-base balance.
You will be closely monitored to detect the first signs of an allergic reaction (such as fever, chills, skin rash, or shortness of breath) when you receive this medicine.
Additional monitoring and tests, such as various blood sample tests, will be performed to ensure that your body is adequately assimilating the administered food.
Nursing staff may also take measures to ensure that your body's fluid and electrolyte needs are met. In addition to this medicine, you may receive additional nutrients (food) to fully meet your needs.
Children
This medicine must not be administered to newborns, infants, or children under two years of age.
Using Lipoflex Plus with Other Medicines
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
Lipoflex Plus may interact with some medicines. Tell your doctor, pharmacist, or nurse if you are taking or receiving any of the following medicines:
? insulin,
? heparin,
? medicines that prevent unwanted blood clotting, such as warfarin or other coumarin derivatives,
? medicines that promote urine flow (diuretics),
? medicines for treating high blood pressure (ACE inhibitors),
? medicines for treating high blood pressure or heart problems (angiotensin-II receptor antagonists),
? medicines used in organ transplantation, such as cyclosporine and tacrolimus,
? medicines for treating inflammation (corticosteroids),
? hormonal preparations that affect your fluid balance (adrenocorticotropic hormone or ACTH).
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. If you are pregnant, you will only receive this medicine if your doctor or pharmacist considers it absolutely necessary for your recovery. There are no data available on the use of Lipoflex Plus in pregnant women.
Breastfeeding is not recommended in mothers treated with parenteral nutrition.
Driving and Using Machines
This medicine is usually administered to patients who are immobilized, e.g., in a hospital or clinic, which excludes the possibility of driving or using machines. However, the medicine itself has no effect on the ability to drive or use machines.
Lipoflex Plus Contains Sodium
This medicine contains 1.150 mg of sodium (the main component of table/cooking salt) in each 1.250 ml bag. This is equivalent to 58% of the maximum recommended daily sodium intake for an adult.
The maximum recommended daily dose of this medicine contains 2.580 mg of sodium (present in table salt). This is equivalent to 129% of the maximum recommended daily sodium intake for an adult.
Consult your doctor or pharmacist if you need one or more bags per day for a prolonged period, especially if you have been advised to follow a low-salt diet (sodium).
3. How to Use Lipoflex Plus
This medicine is administered by intravenous infusion (drip by drip), i.e., through a small tube directly into a vein. This medicine will only be administered through one of your large veins (central veins).
Your doctor or pharmacist will decide what amount of this medicine you need and for how long you will need treatment with it.
Use in Children
This medicine must not be administered to newborns, infants, and children under two years of age.
Your doctor will decide what amount of this medicine your child needs and for how long your child will need treatment with this medicine.
If You Use More Lipoflex Plus Than You Should
If you have received too much of this medicine, you may suffer from the so-called "overload syndrome" and the following symptoms:
? excess fluid and electrolyte disturbances,
? water in your lungs (pulmonary edema),
? loss of amino acids through urine and disturbances in amino acid balance,
? vomiting, nausea,
? chills,
? high blood sugar levels,
? glucose in urine,
? fluid deficit,
? blood that is much more concentrated than normal (hyperosmolality),
? alteration or loss of consciousness due to extremely high blood sugar levels,
? enlarged liver (hepatomegaly) with or without jaundice,
? enlarged spleen (splenomegaly),
? fat deposits in internal organs,
? abnormal liver function test values,
? reduced red blood cell count (anemia),
? reduced white blood cell count (leukopenia),
? reduced platelet count (thrombocytopenia),
? increased immature red blood cells (reticulocytosis),
? rupture of blood cells (hemolysis),
? bleeding or tendency to bleed,
? disturbances of blood coagulation (as can be seen by changes in bleeding time, coagulation time, prothrombin time, etc.),
? fever,
? high levels of fat in the blood,
? loss of consciousness.
If any of the following symptoms occur, the infusion must be stopped immediately.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may be serious. If you experience any of the following side effects, tell your doctor immediately, who will stop administering this medicine to you:
Rare (may affect up to 1 in 1,000 people):
? allergic reactions, such as skin reactions, shortness of breath, swelling of the lips, mouth, and throat, difficulty breathing,
Other side effects include:
Uncommon (may affect up to 1 in 100 people):
? nausea, vomiting, loss of appetite,
Rare (may affect up to 1 in 1,000 people):
? increased tendency of blood to clot,
? bluish discoloration of the skin,
? shortness of breath,
? headache,
? flushing,
? redness of the skin (erythema),
? sweating,
? chills,
? feeling of cold,
? high body temperature,
? drowsiness,
? pain in the chest, back, bones, or lumbar region,
? decreased or increased blood pressure.
Very rare (may affect up to 1 in 10,000 people):
? abnormally high sugar or fat levels in the blood,
? high levels of acidic substances in your blood,
? an excess of lipids can cause overload syndrome; for more information, see the heading "If you use more Lipoflex Plus than you should" in section 3. The symptoms usually disappear when the infusion is stopped.
Frequency not known (cannot be estimated from the available data):
? reduced white blood cell count (leukopenia),
? reduced platelet count (thrombocytopenia),
? disturbances of bile flow (cholestasis).
Reporting of Side Effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website (www.notificaRAM.es)
5. Storage of Lipoflex Plus
Keep this medicine out of the sight and reach of children.
Do not store above 25°C.
Do not freeze. Discard the bag if it has been accidentally frozen.
Keep the bag in the outer packaging to protect it from light.
Do not use this medicine after the expiry date stated on the label. The expiry date is the last day of the month stated.
6. Container Contents and Additional Information
Composition ofLipoflex plus
The active principles of the ready-to-use mixture are:
From the upper chamber (glucose solution) | in 1,000 ml | in 1,250 ml | in 1,875 ml | in 2,500 ml |
Glucose monohydrate | 132.0 g | 165.0 g | 247.5 g | 330.0 g |
equivalent to glucose | 120.0 g | 150.0 g | 225.0 g | 300.0 g |
Sodium dihydrogen phosphate dihydrate | 1.872 g | 2.340 g | 3.510 g | 4.680 g |
Zinc acetate dihydrate | 5.264 mg | 6.580 mg | 9.870 mg | 13.16 mg |
From the middle chamber (fat emulsion) | in 1,000 ml | in 1,250 ml | in 1,875 ml | in 2,500 ml |
Refined soybean oil | 20.00 g | 25.00 g | 37.50 g | 50.00 g |
Medium-chain triglycerides | 20.00 g | 25.00 g | 37.50 g | 50.00 g |
From the lower chamber (amino acid solution) | in 1,000 ml | in 1,250 ml | in 1,875 ml | in 2,500 ml |
Isoleucine | 2.256 g | 2.820 g | 4.230 g | 5.640 g |
Leucine | 3.008 g | 3.760 g | 5.640 g | 7.520 g |
Lysine hydrochloride equivalent to lysine | 2.728 g 2.184 g | 3.410 g 2.729 g | 5.115 g 4.094 g | 6.820 g 5.459 g |
Methionine | 1.880 g | 2.350 g | 3.525 g | 4.700 g |
Phenylalanine | 3.368 g | 4.210 g | 6.315 g | 8.420 g |
Threonine | 1.744 g | 2.180 g | 3.270 g | 4.360 g |
Tryptophan | 0.544 g | 0.680 g | 1.020 g | 1.360 g |
Valine | 2.496 g | 3.120 g | 4.680 g | 6.240 g |
Arginine | 2.592 g | 3.240 g | 4.860 g | 6.480 g |
Histidine hydrochloride monohydrate equivalent to histidine | 1.624 g 1.202 g | 2.030 g 1.503 g | 3.045 g 2.255 g | 4.060 g 3.005 g |
Alanine | 4.656 g | 5.820 g | 8.730 g | 11.64 g |
Aspartic acid | 1.440 g | 1.800 g | 2.700 g | 3.600 g |
Glutamic acid | 3.368 g | 4.210 g | 6.315 g | 8.420 g |
Glycine | 1.584 g | 1.980 g | 2.970 g | 3.960 g |
Proline | 3.264 g | 4.080 g | 6.120 g | 8.160 g |
Serine | 2.880 g | 3.600 g | 5.400 g | 7.200 g |
Sodium hydroxide | 0.781 g | 0.976 g | 1.464 g | 1.952 g |
Sodium chloride | 0.402 g | 0.503 g | 0.755 g | 1.006 g |
Sodium acetate trihydrate | 0.222 g | 0.277 g | 0.416 g | 0.554 g |
Potassium acetate | 2.747 g | 3.434 g | 5.151 g | 6.868 g |
Magnesium acetate tetrahydrate | 0.686 g | 0.858 g | 1.287 g | 1.716 g |
Calcium chloride dihydrate | 0.470 g | 0.588 g | 0.882 g | 1.176 g |
Electrolytes | in 1,000 ml | in 1,250 ml | in 1,875 ml | in 2,500 ml |
Sodium | 40 mmol | 50 mmol | 75 mmol | 100 mmol |
Potassium | 28 mmol | 35 mmol | 52.5 mmol | 70 mmol |
Magnesium | 3.2 mmol | 4.0 mmol | 6.0 mmol | 8.0 mmol |
Calcium | 3.2 mmol | 4.0 mmol | 6.0 mmol | 8.0 mmol |
Zinc | 0.024 mmol | 0.03 mmol | 0.045 mmol | 0.06 mmol |
Chloride | 36 mmol | 45 mmol | 67.5 mmol | 90 mmol |
Acetate | 36 mmol | 45 mmol | 67.5 mmol | 90 mmol |
Phosphate | 12 mmol | 15 mmol | 22.5 mmol | 30 mmol |
Amino acid content | 38 g | 48 g | 72 g | 96 g |
Nitrogen content | 5.4 g | 6.8 g | 10.2 g | 13.7 g |
Carbohydrate content | 120 g | 150 g | 225 g | 300 g |
Lipid content | 40 g | 50 g | 75 g | 100 g |
Energy from lipids | 1,590 kJ (380 kcal) | 1,990 kJ (475 kcal) | 2,985 kJ (715 kcal) | 3,980 kJ (950 kcal) |
Energy from carbohydrates | 2,010 kJ (480 kcal) | 2,510 kJ (600 kcal) | 3,765 kJ (900 kcal) | 5,020 kJ (1,200 kcal) |
Energy from amino acids | 635 kJ (150 kcal) | 800 kJ (190 kcal) | 1,200 kJ (285 kcal) | 1,600 kJ (380 kcal) |
Non-protein energy | 3,600 kJ (860 kcal) | 4,500 kJ (1,075 kcal) | 6,750 kJ (1,615 kcal) | 9,000 kJ (2,150 kcal) |
Total energy | 4,235 kJ (1,010 kcal) | 5,300 kJ (1,265 kcal) | 7,950 kJ (1,900 kcal) | 10,600 kJ (2,530 kcal) |
Osmolality | 1,540 mOsm/kg | 1,540 mOsm/kg | 1,540 mOsm/kg | 1,540 mOsm/kg |
Theoretical osmolality | 1,215 mOsm/l | 1,215 mOsm/l | 1,215 mOsm/l | 1,215 mOsm/l |
pH | 5.0 - 6.0 | 5.0 - 6.0 | 5.0 - 6.0 | 5.0 - 6.0 |
The other components are citric acid monohydrate (for pH adjustment), injectable egg phospholipids, glycerol, sodium oleate, all-rac-alpha-tocopherol, and water for injectable preparations.
Appearance of the product and container contents
The ready-to-use product is an emulsion for infusion, i.e., it is administered through a small tube into a vein.
Lipoflex plus is supplied in flexible multi-chamber bags containing:
- 1,250 ml (500 ml of amino acid solution + 250 ml of fat emulsion + 500 ml of glucose solution),
- 1,875 ml (750 ml of amino acid solution + 375 ml of fat emulsion + 750 ml of glucose solution),
2,500 ml (1,000 ml of amino acid solution + 500 ml of fat emulsion + 1,000 ml of glucose solution).
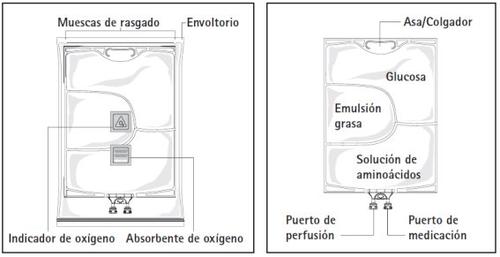
Figure A Figure B
Figure A: The multi-chamber bag is inserted into a protective wrapper. Between the bag and the wrapper are an oxygen absorber and an oxygen indicator; the oxygen absorber wrapper is made of an inert material and contains iron hydroxide.
Figure B: The upper chamber contains a glucose solution, the middle chamber contains a fat emulsion, and the lower chamber contains an amino acid solution.
The glucose and amino acid solutions are clear and colorless to light yellow. The fat emulsion is white and milky.
The upper and middle chambers can be connected to the lower chamber by opening the intermediate seams (peelable seams).
The design of the bag allows for the mixing of amino acids, glucose, lipids, and electrolytes in a single chamber. When the peelable seams are opened, a sterile mixture is formed that creates an emulsion.
The different package sizes are presented in boxes containing five bags.
Package sizes: 5 x 1,250 ml, 5 x 1,875 ml, and 5 x 2,500 ml
Only some package sizes may be marketed.
Marketing authorization holder:
- Braun Melsungen AG
Carl-Braun-Str. 1 Postal address:
34212 Melsungen, Germany 34209 Melsungen, Germany
Tel.: +49-(0)-5661-71-0
Fax: +49-(0)-5661-71-4567
Manufacturer
- Braun Melsungen AG
Am Schwerzelshof 1
34 212 Melsungen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder
- Braun Medical, S.A.
Ctra. de Terrassa, 121
08191 Rubí, Spain
This medication is authorized in the member states of the European Economic Areaand in the United Kingdom (Northern Ireland)with the following names:
Austria Nutriflex Lipid plus B.Braun
Belgium Nutriflex Lipid plus, 38 g/l AA + 120 g/l G, emulsie voor infusie
Denmark Lipoflex Plus
Finland Nutriflex Lipid 38/120/40
France LIPOFLEX LIPIDE G120/N 5,4 /E, émulsion pour perfusion
Germany Nutriflex Lipid 38/120 plus
Iceland Nutriflex Lipid 38/120 plus
Italy Lipoflex AA38/G120
Luxembourg Nutriflex Lipid 38/120 plus
Netherlands Nutriflex Lipid plus, 38 g/l + 120 g/l, emulsie voor infusie
Norway Lipoflexplus
Poland Lipoflex plus
Romania Lipoflex plus emulsie perfuzabila
Spain Lipoflex plus emulsión para perfusión
Sweden Nutriflex Lipid 38/120/40
United Kingdom
(Northern Ireland) Lipoflex plus emulsion for infusion
Date of the last revision of this prospectus:
09/2023
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
______________________________________________________________________________
This information is intended only for healthcare professionals:
No special conditions are required for disposal.
Parenteral nutrition products should be visually inspected before use to detect damage, color changes, and emulsion instability.
Do not use damaged bags. The wrapper, main bag, and peelable seams between the chambers must be intact. Use only if the amino acid and glucose solutions are clear and colorless to light yellow and if the lipid emulsion is homogeneous and has a white, milky color. Do not use if the solutions contain particles.
After mixing the three chambers, do not use if the emulsion shows a color change or signs of phase separation (oil droplets, oil layer). Stop the infusion immediately in case of a color change in the emulsion or signs of phase separation.
Before opening the wrapper, check the color of the oxygen indicator (see Figure A). Do not use if the oxygen indicator changes to a pink color. Use only if the oxygen indicator is yellow.
Preparation of the mixed emulsion
A strict adherence to aseptic handling principles is required.
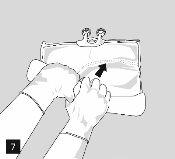
For opening: open the wrapper starting from the tear-off notches (Fig. 1). Remove the bag from its protective wrapper. Discard the wrapper, oxygen indicator, and oxygen absorber.
Visually inspect the main bag for leaks. Bags with leaks must be discarded, as their sterility cannot be guaranteed.
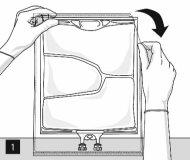
Mixing the bag and adding additives
To open and mix the chambers sequentially, roll the bag with both hands, first opening the peelable seam that separates the upper chamber (glucose) from the lower chamber (amino acids) (Fig. 2).
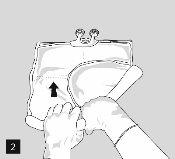
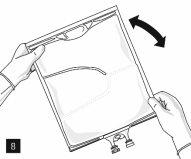
After removing the aluminum foil (Fig. 3), it is possible to add compatible hydrophilic additives through the medication port (Fig. 4) to the clear aqueous solutions. Mix the bag contents well (Fig. 5) and visually inspect the mixture for precipitates (Fig. 6). The solution should only be used if it is clear.
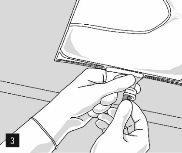
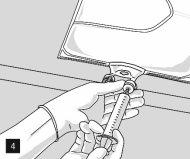
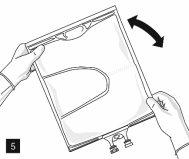
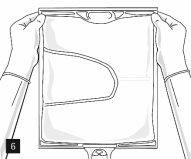
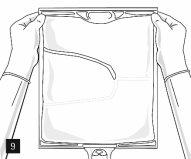
Then, continue to apply pressure so that the peelable seam that separates the middle chamber (lipids) from the lower chamber (Fig. 7) opens. The mixture is a homogeneous oil-in-water emulsion with a white, milky color. Once all the chambers have been mixed, it is possible to add compatible additives through the medication port (Fig. 4). Mix the bag contents well (Fig. 8) and visually inspect the mixture (Fig. 9).
The manufacturer may provide, upon request, data on the compatibility of different additives (e.g., electrolytes, trace elements, vitamins) and the corresponding validity periods of these mixtures.
Preparation for infusion
The emulsion should always be brought to room temperature before infusion.
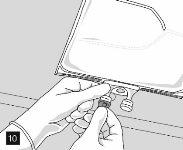
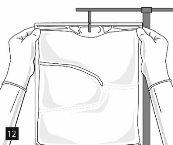
Remove the aluminum foil (Fig. 10) from the infusion port and connect the infusion equipment (Fig. 11). Use a non-ventilated infusion set or close the air vent if a ventilated set is used. Hang the bag from an infusion hook (Fig. 12) and perform the infusion according to the standard technique.
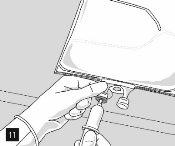
For single use. The package and unused residues should be disposed of after use.
Do not reconnect partially used packages.
If filters are used, they must be lipid-permeable (pore size ≥ 1.2 μm).
Validity period after removing the protective wrapper and after mixing the bag contents
Chemical and physicochemical stability of the amino acid, glucose, and lipid mixture has been demonstrated for 7 days at 2-8°C and for 2 additional days at 25°C.
Validity period after additional mixing of compatible additives
From a microbiological point of view, the product should be used immediately after the additional mixing of additives. Otherwise, the storage times during use and the conditions prior to use are the responsibility of the user.
The emulsion should be used immediately after opening the package.
The recommended duration for the infusion of a parenteral nutrition bag is a maximum of 24 hours.
This medication should not be mixed with other medications whose compatibility has not been documented.
This medication should not be administered simultaneously with blood in the same infusion equipment due to the risk of pseudoagglutination.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LIPOFLEX PLUS EMULSION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for LIPOFLEX PLUS EMULSION FOR INFUSION
Discuss questions about LIPOFLEX PLUS EMULSION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















