
LEVONORGESTREL/ETHINYLESTRADIOL CINFALAB 0.1 mg/0.02 mg FILM-COATED TABLETS

How to use LEVONORGESTREL/ETHINYLESTRADIOL CINFALAB 0.1 mg/0.02 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG and what is it used for
- What you need to know before taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
- Bleeding between periods
- What to do if there is no bleeding during the treatment-free week
- How to take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
- Possible side effects
- Storage of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
- Package contents and additional information
Introduction
Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
Read the entire leaflet carefully before starting to take this medication, as it contains important information for you.
If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4. |
Contents of the leaflet:
- What is levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG and what is it used for.
- What you need to know before taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG.
- Do not use levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg.
- Warnings and precautions
- Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg and thrombosis
- Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg and cancer
- Bleeding between periods
- What to do if there is no bleeding during the treatment-free week
- Use of other medications
- Lab tests
- Pregnancy, breastfeeding, and fertility
- Driving and using machines
- Important information about some of the ingredients of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg
- How to take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG.
- When can you start with the first strip
- If you take more levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg than you should
- If you forget to take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg What to do in case of vomiting or severe diarrhea
- Delayed menstrual period: what you should know
- Change of the first day of your menstrual period: what you should know
If you want to stop taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg
- Possible side effects.
- Storage of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
- Package contents and additional information.
1. What is levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG and what is it used for
- Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg is a contraceptive pill used to prevent pregnancy.
- Each pink tablet contains a small amount of two different female hormones, called levonorgestrel and ethinylestradiol.
- Contraceptive pills that contain two hormones are known as "combined pills".
2. What you need to know before taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
General considerations
Before you can start taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg, your doctor will ask you some questions about your medical history and that of your close relatives. Your doctor will also measure your blood pressure and, depending on your personal situation, may perform other tests.
This leaflet describes several situations in which you should stop using levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg or in which the reliability of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg may be decreased. In these situations, you should not have sexual intercourse or, if you do, you should use other non-hormonal contraceptive methods (e.g., a condom or another barrier method). Do not use the rhythm or temperature methods. These methods are unreliable, as levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg alters the monthly changes in body temperature and cervical mucus.
Like other hormonal contraceptives, levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg does not protect against HIV (AIDS) or any other sexually transmitted disease.
Do not use levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
Do not take levonorgestrel/ethinylestradiol cinfalab if you have hepatitis C and are taking medications that contain ombitasvir/paritaprevir/ritonavir, dasabuvir, glecaprevir/pibrentasvir, or sofosbuvir/velpatasvir/voxilaprevir (see also the section "Taking levonorgestrel/ethinylestradiol cinfalab with other medications").
- If you are allergic to levonorgestrel or ethinylestradiol, or to any of the other ingredients of levonorgestrel/ethinylestradiol cinfalab 100/20 micrograms (listed in section 6). This allergy can be recognized by the appearance of itching, rash, or inflammation.
- If you have (or have had in the past) a blood clot (thrombosis) in a blood vessel of the leg, lungs (embolism), or other organs.
- If you have (or have had in the past) a heart attack or stroke.
- If you have (or have had in the past) a disease that may predict a heart attack (e.g., angina pectoris, which causes severe chest pain) or a stroke (e.g., a small, transient stroke without residual effects).
- If you have a disease that could increase the risk of thrombosis in the arteries. These warnings apply to the following situations:
- diabetes with blood vessel damage
- very high blood pressure
- very high levels of fat in the blood (cholesterol or triglycerides)
- If you have a blood coagulation disorder (e.g., protein C deficiency).
- If you have (or have had) a certain type of migraine (with so-called focal neurological symptoms).
- If you have (or have had) pancreatitis (inflammation of the pancreas).
- If you have or have had liver disease in the past and if your liver function is still not normal.
- If you have or have had a liver tumor.
- If you have (or have had) or if there is a suspicion of breast cancer or cancer of the genital organs
- If you have unexplained vaginal bleeding.
- If you have not had your period for several months without a known cause.
Warnings and precautions
Consult your doctor or pharmacist before starting to take levonorgestrel/ethinylestradiol cinfalab.
In some situations, you will need to take special precautions when using levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg or any other combined hormonal contraceptive, and sometimes you will need to see your doctor periodically. If you are in any of the following situations, you should inform your doctor before starting to use levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg. Additionally, you should consult your doctor if any of the following situations appear or worsen while using levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg:
- If a close relative has or has had breast cancer.
- If you have a liver or gallbladder disease.
- If you have diabetes.
- If you have depression.
- If you have Crohn's disease or ulcerative colitis (inflammatory bowel disease).
- If you have hemolytic uremic syndrome (HUS), a blood disorder that causes kidney damage.
- If you have sickle cell anemia (a hereditary disease of the red blood cells).
- If you have epilepsy.
- If you have systemic lupus erythematosus (SLE, an immune system disorder).
- If you have a disease that first appeared during pregnancy or during previous use of sex hormones (e.g., loss of hearing, porphyria [a blood disease], gestational herpes [a skin rash with blisters that appears during pregnancy], Sydenham's chorea [a nerve disease in which sudden movements of the body occur]).
- If you have or have had chloasma (brownish-yellow pigmented patches, known as "pregnancy patches", especially on the face). If so, avoid direct exposure to sunlight or ultraviolet light.
- If you experience symptoms of angioedema, such as swelling of the face, tongue, and/or throat, and/or difficulty swallowing or urticaria with possible difficulty breathing, contact a doctor immediately. Products containing estrogens may cause or worsen the symptoms of hereditary and acquired angioedema.
Psychiatric disorders:
Some women who use hormonal contraceptives like levonorgestrel/ethinylestradiol cinfalab have reported depression or a depressed mood. Depression can be severe and sometimes may induce suicidal thoughts. If you experience mood changes and depressive symptoms, contact your doctor for additional medical advice as soon as possible.
Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG and thrombosis
Venous thrombosis
The use of any combined pill, including levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg, increases the risk of a woman developing a venous thrombosis (formation of a blood clot in a blood vessel) compared to a woman who does not take any contraceptive pill.
The risk of venous thrombosis increases in users of combined pills:
- With age,
- If you are overweight,
- If any of your close relatives have had a blood clot (thrombosis) in the leg, lung, or other organ at a young age,
- If you need to undergo surgery (surgery), a prolonged period of immobilization, or if you have had a serious accident. It is important that you discuss with your doctor that you are using levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg, as you may need to interrupt treatment. Your doctor will tell you when you can start taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg again. Normally, this will be within 2 weeks after your recovery.
Arterial thrombosis
The use of combined pills has been associated with an increased risk of arterial thrombosis (blockage of an artery), for example, in the blood vessels of the heart (heart attack) or brain (stroke).
The risk of arterial thrombosis increases in users of combined pills:
- If you smoke. You are strongly advised to stop smoking when using levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg, especially if you are over 35 years old.
- If you have high levels of fat in your blood (cholesterol or triglycerides).
- If you are overweight.
- If any of your close relatives have had a heart attack or stroke at a young age.
- If you have high blood pressure.
- If you have migraines.
- If you have heart problems (a valvular disorder or a heart rhythm disturbance).
Stop taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg and contact your doctor immediately if you notice signs of possible thrombosis, such as:
|
Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG and cancer
There have been cases of breast cancer with a slightly higher frequency in women taking contraceptive pills, but it is not known if this is due to the treatment. For example, it could be that more tumors are detected in women taking combined pills because they are examined by their doctor more frequently. The occurrence of breast tumors has been gradually lower after stopping the use of combined hormonal contraceptives. It is essential to regularly check your breasts, and you should contact your doctor if you notice any lump.
In rare cases, benign liver tumors and even rarer cases of malignant liver tumors have been described in users of contraceptive pills. Contact your doctor if you notice unusual, severe abdominal pain.
Bleeding between periods
During the first few months of treatment with levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg, you may experience unexpected bleeding (bleeding outside of the treatment-free week). If this bleeding lasts for more than a few months or starts after some months, your doctor should investigate the cause.
What to do if there is no bleeding during the treatment-free week
If you have taken all the tablets correctly, have not vomited, and have not had severe diarrhea, and have not taken any other medications, it is very unlikely that you are pregnant.
If the expected bleeding does not occur in two consecutive instances, you may be pregnant. Contact your doctor immediately. Do not start taking the next strip until you are sure you are not pregnant.
Use of levonorgestrel/ethinylestradiol cinfalab with other medications
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medications or herbal remedies.
Do not take levonorgestrel/ethinylestradiol cinfalab if you have hepatitis C and are taking medications that contain ombitasvir/paritaprevir/ritonavir, dasabuvir, glecaprevir/pibrentasvir, or sofosbuvir/velpatasvir/voxilaprevir, as these medications may cause increased liver enzyme test results (elevated liver enzyme ALT).
Your doctor will prescribe another type of contraceptive before starting treatment with these medications.
Levonorgestrel/ethinylestradiol cinfalab can be used again approximately 2 weeks after the end of this treatment. See the section "Do not use levonorgestrel/ethinylestradiol cinfalab".
Additionally, inform any other doctor or dentist who prescribes you other medications (or the pharmacist who dispenses them) that you are using levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg.They may tell you if you need to use additional contraceptive measures (e.g., condoms) and, if so, for how long.
Some medications reduce the effectiveness of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg in preventing pregnancy or may cause unexpected bleeding.
These include medications used to treat epilepsy (e.g., primidone, phenytoin, barbiturates, carbamazepine, or oxcarbazepine) and tuberculosis (e.g., rifampicin) or HIV infections (e.g., ritonavir) or other infectious diseases (such as griseofulvin, ampicillin, or tetracycline), which increase intestinal motility (such as metoclopramide), and the herbal remedy St. John's Wort.
If you want to use herbal remedies that contain hypericum while taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg, you should consult your doctor first.
Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg reduces the effectiveness of other medications, such as those containing cyclosporin, or the antiepileptic lamotrigine (which may increase the frequency of seizures).
Consult your doctor or pharmacist before using any medication.
Lab tests
If you need a blood test, inform your doctor or the laboratory staff that you are taking the pill, as oral contraceptives affect the results of some tests.
Pregnancy, breastfeeding, and fertility
If you are pregnant, you should not take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg. If you become pregnant while taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg, you should stop taking it immediately and contact your doctor.
Consult your doctor or pharmacist before using any medication.
Breastfeeding
Generally, it is not recommended to use levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg when breastfeeding. You should consult your doctor if you want to take the pill while breastfeeding.
Consult your doctor or pharmacist before using any medication.
Driving and using machines
There is no information to suggest that the use of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg affects the ability to drive or use machines.
Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets contain lactose.
This medication contains lactose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
3. How to take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
Follow exactly the administration instructions of this medication indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Take one levonorgestrel/ethinylestradiol cinfalab0.1 mg/0.02 mg tablet every day, if necessary with a small amount of water. You should take the tablets every day, more or less at the same time.
The strip contains 21 tablets, each marked with a day of the week. You should start by taking the tablet marked with the correct day of the week. Follow the direction of the arrows on the strip. Take one tablet every day until you have finished the 21 tablets. After that, do not take any more tablets for 7 days.
During those 7 days without tablets (known as interruption or treatment-free week) you should start bleeding. This is called "withdrawal bleeding" and usually starts on the 2nd or 3rd day of the week.
On the 8th day after taking the last levonorgestrel/ethinylestradiol cinfalab0.1 mg/0.02 mg tablet (i.e., after the 7-day treatment-free week), start taking the next strip, even if the bleeding has not stopped.
This means you should start taking the next strip on the same day of the week, and the withdrawal bleeding should appear on the same days every month.
If you use levonorgestrel/ethinylestradiol cinfalab0.1 mg/0.02 mg
When can you start with the first strip
- If you have not used a hormonal contraceptive in the previous month.
Start with levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg on the first day of your cycle (which is the first day of your menstruation). If you start taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg on the first day of your menstruation, you will be immediately protected against pregnancy. You can also start on days 2-5 of your cycle, but in that case, you must use extra protective measures (e.g., a condom) during the first 7 days.
- Changing from another combined hormonal contraceptive or a vaginal ring or a combined contraceptive patch
You can start taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg on the day after the tablet-free period of the pill you just finished (or after the last inactive tablet of your previous pill).
When changing from a vaginal ring or a combined contraceptive patch, follow your doctor's advice.
- Changing from a progestogen-only method (progestogen-only pill or injection, implant, or IUD releasing progestogen).
You can change any day from the progestogen-only pill (if you used an implant or IUD, on the day of its removal, and if you received the progestogen by injection, on the date the next injection was due), but in all cases, you must apply additional protective measures (e.g., a condom) during the first 7 days you take the new pills.
- After an abortion or miscarriage.
Follow your doctor's instructions.
- After having a child.
After having a child,you can start taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg between 21 and 28 days later. If you start later than day 28, you must use a barrier method (e.g., a condom) during the first 7 days of taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg.
If, after having a child, you have had sexual intercourse before starting to take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg again, you must first check that you are not pregnant or wait until your next menstrual period.
Ask your doctor for advice if you are not sure when to start.
- If you are breastfeeding and want to start taking levonorgestrel/ethinylestradiol cinfalab0.1 mg/0.02 mg again after having a child
Read the section on "Breastfeeding".
If you take more levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg tablets than you should
There are no reports of harmful effects from taking too many levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg tablets. If you take several tablets at once, you may have symptoms of nausea and vomiting. Young girls may have vaginal bleeding.
If you have taken too many levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg tablets, or if you discover that your child has taken some, consult your doctor or pharmacist.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg tablets
- If it has been less than 12 hourssince you forgot the tablet, your protection against pregnancy will not be affected. You can still take the tablet as soon as you remember and then take the following tablets at the usual time.
- If it has been more than 12 hourssince you forgot the tablet, your protection against pregnancy may be reduced. The more tablets you forget to take, the greater the risk that your protection against pregnancy will be reduced.
The risk of incomplete protection against pregnancy is higher if you forget to take a tablet at the beginning or end of a strip.
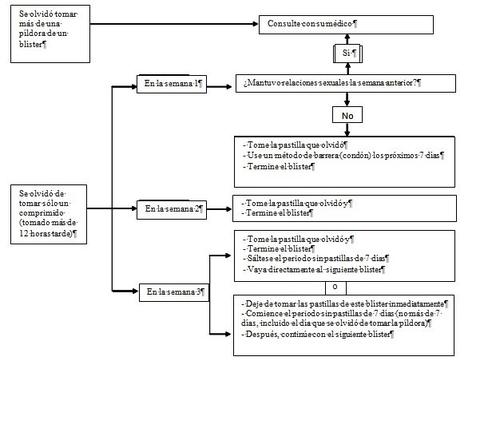
Therefore, you should follow these rules (see also the diagram below):
- More than 1 forgotten tablet in this strip
Consult your doctor.
- 1 forgotten tablet in week 1
Take the forgotten tablet as soon as you remember, even if it means taking two tablets at the same time. Take the tablets afterwards at the usual time and take extra precautionsfor the next 7 days, for example, a condom. If you have had sexual intercourse in the week before the missed tablet, or if you forgot to start a new strip after the tablet-free period, you should be aware that there is a risk of pregnancy. In that case, consult your doctor.
- 1 forgotten tablet in week 2
Take the forgotten tablet as soon as you remember, even if it means taking two tablets at the same time. Take the tablets afterwards at the usual time. Your protection against pregnancy will not be reduced, and you will not need to take extra precautions.
1 forgotten tablet in week 3
You can choose between 2 options:
- Take the forgotten tablet as soon as you remember, even if it means taking two tablets at the same time.
Take the tablets afterwards at the usual time. Instead of the tablet-free period, start the next strip directly.
It is likely that you will have a menstrual period (withdrawal bleeding) after finishing the second strip, but you may also have spotting or intermenstrual bleeding when taking the second strip.
- You can also stop taking that strip and go directly to the 7-day tablet-free period (note the day you forgot to take your tablet). If you want to start a new strip on a specific day, shorten the tablet-free period to less than 7 days.
If you follow one of these two recommendations, you will remain protected against pregnancy.
If you forgot to take any of the tablets in a strip and do not have bleeding during the first tablet-free period, it could mean that you are pregnant. You should contact your doctor before continuing with the next strip.
What to do in case of vomiting or severe diarrhea
If you vomit within 3-4 hours after taking the tablet or if you have significant diarrhea, there is a risk that the active ingredients of the tablet will not be fully absorbed into your body. The situation is similar to when you forget to take a tablet. After vomiting or having diarrhea, you should take another tablet from the reserve strip as soon as possible. If possible, take it within 12 hoursof the time you would normally take your tablet. If it is not possible, or if more than 12 hours have passed, you should follow the advice included in "If you forget to take levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg.
Delayed menstrual period: what you should know
Although it is not recommended, it is possible to delay your menstrual period (withdrawal bleeding) by taking a new strip of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg directly instead of the tablet-free period, until the end of the second strip. You may have spotting (drops or spots of blood) or intermenstrual bleeding while using the second strip. After the usual 7-day tablet-free period, continue with the next strip.
You may need to ask your doctor for advice before deciding whether to delay your menstrual cycle.
Changing the first day of your period: what you should know
If you take the tablets according to the instructions, your menstrual period or withdrawal bleeding will start during the tablet-free week. If you need to change this day, do so by shortening the tablet-free period (but never lengthening it). For example, if your tablet-free period starts on a Friday and you want to change to a Tuesday (3 days earlier), you should start a new strip 3 days earlier than usual. If you shorten the tablet-free period significantly (e.g., 3 days or less), you may not have any bleeding during this tablet-free period. Afterwards, you may have spotting (drops or spots of blood) or intermenstrual bleeding.
If you are not sure what to do, ask your doctor for advice.
If you want to stop taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg tablets
You can stop taking levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg whenever you want. If you do not want to become pregnant, ask your doctor for advice on other reliable methods of birth control.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg can have side effects, although not everyone gets them.
Always inform your doctor if you experience any side effects, especially if the side effect is intense or persistent, or if you notice any change in your health that you think may be due to the pill.
Several side effects related to the use of the pill are described in the sections "levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg and thrombosis" and "levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg and cancer". Read those paragraphs for more information and consult your doctor immediately if necessary.
- Common side effects (may affect up to 1 in 10 women): headache, mood changes (including depression), nausea, abdominal pain, breast tenderness, breast sensitivity, weight gain, skin rash.
- Uncommon side effects (may affect up to 1 in 100 women): vomiting, diarrhea, fluid retention or edema, migraine, loss of libido, breast enlargement, hives.
- Rare side effects (may affect up to 1 in 1,000 women): eye irritation with contact lens use, hypersensitivity, weight loss, breast secretion, vaginal discharge, increased libido, erythema nodosum (nodules on the legs), erythema multiforme (skin lesions).
Contact a doctor immediately if you experience any of the following symptoms of angioedema: swelling of the face, tongue, and/or throat, and/or difficulty swallowing or hives with possible difficulty breathing (see also section "Warnings and precautions").
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets EFG
Keep this medicine out of the sight and reach of children.
Do not store above 30°C.
Do not use levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg after the expiry date stated on the outer packaging and on the blister after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medication in the SIGRE collection point at your usual pharmacy. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Composition of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets
The active ingredients are levonorgestrel and ethinylestradiol.
Each tablet contains 0.1 mg of levonorgestrel and 0.02 mg of ethinylestradiol. The other ingredients (excipients) are anhydrous lactose, Povidone K-30 (E1201), magnesium stearate (E572), and Opadry II pink [polyvinyl alcohol, talc (E553b), titanium dioxide (E171), polyethylene glycol 3350, aluminum lake red (E129), lecithin (E322), iron oxide red (E172), and aluminum lake blue (E1329)].
Appearance of levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg film-coated tablets and package contents
- Each film-coated tablet is pink and round.
- Levonorgestrel/ethinylestradiol cinfalab 0.1 mg/0.02 mg is marketed in strips (blister packs) of 21 tablets.
- The package sizes are 1, 3, or 6 strips, and each strip contains 21 tablets. Not all package sizes may be marketed.
Marketing authorization holder
Laboratorios Cinfa, S.A.
Carretera Olaz-Chipi, 10. Polígono Industrial Areta.
31620 Huarte (Navarra) - Spain
Manufacturer
Laboratorios León Farma, S.A.
Pol. Ind. Navatejera;
La Vallina s/n;
24008-Villaquilambre, León
Spain
This leaflet was approved in:October 2022
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEVONORGESTREL/ETHINYLESTRADIOL CINFALAB 0.1 mg/0.02 mg FILM-COATED TABLETSDosage form: TABLET, 0.1 mg/0.02 mgActive substance: levonorgestrel and ethinylestradiolManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: TABLET, 0.1 mg/0.02 mgActive substance: levonorgestrel and ethinylestradiolManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: TABLET, 0.10 mg/0.02 mgActive substance: levonorgestrel and ethinylestradiolManufacturer: Sandoz Farmaceutica S.A.Prescription required
Online doctors for LEVONORGESTREL/ETHINYLESTRADIOL CINFALAB 0.1 mg/0.02 mg FILM-COATED TABLETS
Discuss questions about LEVONORGESTREL/ETHINYLESTRADIOL CINFALAB 0.1 mg/0.02 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






