
LEVETIRACETAM RATIOPHARM 100 mg/ml ORAL SOLUTION

How to use LEVETIRACETAM RATIOPHARM 100 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Levetiracetam ratiopharm 100 mg/ml Oral Solution EFG
levetiracetam
Read the entire package leaflet carefully before you or your child starts taking this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only and you should not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Levetiracetam ratiopharm and what is it used for
- What you need to know before taking Levetiracetam ratiopharm
- How to take Levetiracetam ratiopharm
- Possible side effects
- Storage of Levetiracetam ratiopharm
- Contents of the pack and further information
1. What is Levetiracetam ratiopharm and what is it used for
Levetiracetam is an antiepileptic medicine (a medicine used to treat seizures in epilepsy).
Levetiracetam ratiopharm is used:
- alone in adults and adolescents from 16 years of age with newly diagnosed epilepsy, to treat a form of epilepsy. Epilepsy is a disease where patients have seizures (fits). Levetiracetam is used for the form of epilepsy in which the seizures initially affect only one side of the brain, but may then spread to wider areas on both sides of the brain (partial onset seizures with or without secondary generalisation). Your doctor has prescribed levetiracetam to reduce the number of seizures.
- in combination with other antiepileptic medicines to treat:
- partial onset seizures with or without secondary generalisation in adults, adolescents, children and infants from 1 month of age
- myoclonic seizures (short, shock-like jerks of a muscle or group of muscles) in adults and adolescents from 12 years of age with juvenile myoclonic epilepsy
- primary generalised tonic-clonic seizures (major fits, including loss of consciousness) in adults and adolescents from 12 years of age with idiopathic generalised epilepsy (a type of epilepsy that is thought to have a genetic cause).
2. What you need to know before taking Levetiracetam ratiopharm
Do not take Levetiracetam ratiopharm
- if you are allergic to levetiracetam, to pyrrolidone derivatives or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before starting to use Levetiracetam ratiopharm
- if you have kidney problems, follow your doctor's instructions. He/She will decide if you need to adjust your dose.
- if you notice any decrease in your child's growth or an unexpected delay in puberty, contact your doctor.
- a small number of people taking antiepileptics such as Levetiracetam ratiopharm have had thoughts of harming themselves or suicide. If you have any symptoms of depression and/or suicidal thoughts, contact your doctor.
- if you have a medical history or a family history of irregular heart rhythm (visible on an electrocardiogram), or if you have a disease and/or take a treatment that makes you prone to cardiac arrhythmias or electrolyte imbalances.
Tell your doctor or pharmacist if any of the following side effects get worse or last more than a few days:
- abnormal thoughts, feeling irritable or reacting more aggressively than usual or if you or your family and friends notice important changes in mood or behaviour.
- worsening of epilepsy
In rare cases, epileptic seizures may worsen or occur more frequently, mainly during the first month after starting treatment or after increasing the dose. If you experience any of these new symptoms while taking Levetiracetam ratiopharm, see a doctor as soon as possible.
Children and adolescents
- Monotherapy with Levetiracetam ratiopharm (alone) is not indicated in children and adolescents under 16 years.
Using Levetiracetam ratiopharm with other medicines
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Do not take macrogol (a laxative medicine) during the hour before and the hour after taking levetiracetam, as it may reduce its effect.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Levetiracetam should only be used during pregnancy if, after a careful evaluation, your doctor considers it necessary.
Do not stop your treatment without consulting your doctor.
The risk of birth defects for your baby cannot be completely excluded.
Breastfeeding is not recommended during treatment.
Driving and using machines
Levetiracetam ratiopharm may affect your ability to drive or use tools or machinery, as it may cause drowsiness. This is more likely at the start of treatment or when the dose is increased. You should not drive or use machinery until it is established that your ability to perform these activities is not affected.
Levetiracetam ratiopharm contains methyl parahydroxybenzoate, propyl parahydroxybenzoate, potassium and sodium
Levetiracetam ratiopharm oral solution may cause allergic reactions (which may be delayed) because it contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216).
This medicine contains 1.2 mmol (or 46.65 mg) of potassium per 15 ml, which should be taken into account in patients with renal insufficiency or in patients on a low potassium diet.
This medicine contains less than 1 mmol of sodium (23 mg) per 15 ml; this is essentially “sodium-free”.
3. How to take Levetiracetam ratiopharm
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
Levetiracetam ratiopharm should be taken twice daily, once in the morning and once in the evening, approximately at the same time each day.
Take the oral solution as instructed by your doctor.
Monotherapy (from 16 years of age)
Adults (≥18 years) and adolescents (from 16 years of age):
Measure the appropriate dose using the 10 ml syringe included in the pack for patients from 4 years of age onwards.
Recommended dose: Levetiracetam ratiopharm is taken twice daily, divided into two equal doses, each of 5 ml (500 mg) to 15 ml (1,500 mg).
When starting Levetiracetam ratiopharm, your doctor will prescribe a lower dosefor two weeks before giving you the lowest daily dose.
Concomitant therapy
Dose in adults and adolescents (12 to 17 years):
Measure the appropriate dose using the 10 ml syringe included in the pack for patients from 4 years of age onwards.
Recommended dose: Levetiracetam ratiopharm is taken twice daily, divided into two equal doses, each of 5 ml (500 mg) to 15 ml (1,500 mg).
Dose in children from 6 months of age onwards:
Your doctor will prescribe the most appropriate pharmaceutical form of Levetiracetam ratiopharm according to the age, weight and dose.
For children from 6 months to 4 years, measure the appropriate dose using the 3 ml syringe included in the pack.
For children over 4 years, measure the appropriate dose using the 10 ml syringe included in the pack.
Recommended dose: Levetiracetam ratiopharm is taken twice daily, divided into two equal doses, each of 0.1 ml (10 mg) to 0.3 ml (30 mg) per kg of the child's body weight (see examples of doses in the table below).
Dose in children from 6 months of age onwards:
Weight | Initial dose: 0.1 ml/kg twice daily | Maximum dose: 0.3 ml/kg twice daily |
6 kg | 0.6 ml twice daily | 1.8 ml twice daily |
8 kg | 0.8 ml twice daily | 2.4 ml twice daily |
10 kg | 1 ml twice daily | 3 ml twice daily |
15 kg | 1.5 ml twice daily | 4.5 ml twice daily |
20 kg | 2 ml twice daily | 6 ml twice daily |
25 kg | 2.5 ml twice daily | 7.5 ml twice daily |
From 50 kg | 5 ml twice daily | 15 ml twice daily |
Dosing in infants (from 1 month to less than 6 months):
For infants from 1 month to less than 6 months, measure the appropriate dose using the 1 ml syringe included in the pack.
Recommended dose: Levetiracetam ratiopharm is taken twice daily, divided into two equal doses, each of 0.07 ml (7 mg) to 0.21 ml (21 mg) per kg of the infant's body weight (see examples of doses in the table below).
Dosing in infants (from 1 month to less than 6 months):
Weight | Initial dose: 0.07 ml/kg twice daily | Maximum dose: 0.21 ml/kg twice daily |
4 kg | 0.3 ml twice daily | 0.85 ml twice daily |
5 kg | 0.35 ml twice daily | 1.05 ml twice daily |
6 kg | 0.45 ml twice daily | 1.25 ml twice daily |
7 kg | 0.5 ml twice daily | 1.5 ml twice daily |
Method of administration
After measuring the correct dose with an appropriate syringe, Levetiracetam ratiopharm can be taken by diluting the oral solution in a glass of water or in a baby's bottle. You can take Levetiracetam ratiopharm with or without food. After oral administration of levetiracetam, its bitter taste may be noticed.
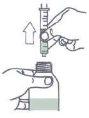
Instructions for correct administration:
- Open the bottle: press the cap and unscrew in the opposite direction to the arrow.
- Take the syringe and insert it into the bottle opening.
For this, the plunger must be completely inside the syringe (figure)
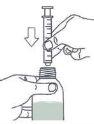
- Hold the bottle and syringe firmly together. Turn the bottle and syringe
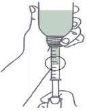
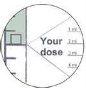
- Fill the syringe by pulling the plunger to the mark corresponding to the dose in milliliters (ml) prescribed by your doctor.
- You can read the amount corresponding to the milliliters at the beginning of the thicker part of the plunger (figure).
- If bubbles appear, press the plunger back into the syringe and fill the syringe again slowly.
- Move the bottle and syringe back to the initial position.
- Remove the filled syringe from the bottle (figure).
- Empty the contents of the syringe into a glass of water by pressing the syringe plunger (figure).
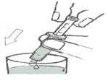
- Close the bottle with the plastic screw cap after each use.
- Drink the entire contents of the glass.
- Wash the syringe after each use with clear water by filling and emptying the syringe repeatedly.
Duration of treatment
- Levetiracetam ratiopharm is used as a chronic treatment. You should continue treatment with Levetiracetam ratiopharm for the time indicated by your doctor.
- Do not stop your treatment without your doctor's recommendation, as your seizures may increase.
If you take more Levetiracetam ratiopharm than you should
Possible side effects of an overdose of Levetiracetam ratiopharm are drowsiness, agitation, aggression, decreased alertness, respiratory inhibition and coma.
Contact your doctor if you have taken more Levetiracetam ratiopharm than you should. Your doctor will establish the best possible treatment for the overdose.
If you forget to take Levetiracetam ratiopharm
Contact your doctor if you have missed one or more doses.
Do not take a double dose to make up for forgotten doses.
If you stop treatment with Levetiracetam ratiopharm
Stopping treatment with Levetiracetam ratiopharm should be done gradually to avoid an increase in seizures. If your doctor decides to stop your treatment with Levetiracetam ratiopharm, he/she will give you instructions for gradual withdrawal of Levetiracetam ratiopharm.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, Levetiracetam ratiopharm can cause adverse effects, although not all people suffer from them.
Tell your doctor immediately or go to the emergency department of your nearest hospital if you experience:
- weakness, dizziness, or difficulty breathing, as these can be signs of a severe allergic reaction (anaphylactic)
- swelling of the face, lips, tongue, or throat (Quincke's edema)
- flu-like symptoms and rash on the face followed by a prolonged rash with high temperature, elevated liver enzyme levels in blood tests, and an increase in a type of white blood cells (eosinophilia) and enlarged lymph nodes (Drug Hypersensitivity Reaction with Eosinophilia and Systemic Symptoms [DRESS])
- symptoms such as low urine output, fatigue, nausea, vomiting, confusion, and swelling of legs, ankles, or feet, as these may be signs of sudden decreased renal function
- a skin rash that can form blisters and may appear as small targets (dark central spots surrounded by a paler area, with a dark ring around the edge) (erythema multiforme)
- a widespread rash with blisters and skin peeling, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome)
- a more severe form of rash that causes skin peeling on more than 30% of the body surface (toxic epidermal necrolysis)
- signs of severe mental changes or if someone around you notices signs of confusion, drowsiness (drowsiness), amnesia (memory loss), memory impairment (memory loss), abnormal behavior, or other neurological signs including involuntary or uncontrolled movements. These can be symptoms of encephalopathy.
The most frequently reported adverse effects are nasopharyngitis, drowsiness (feeling of sleep), headache, fatigue, and dizziness. Adverse effects such as drowsiness, weakness, and dizziness may be more frequent when treatment is initiated or the dose is increased.
However, these adverse effects should decrease over time.
Very common:may affect more than 1 in 10 people
- nasopharyngitis;
- drowsiness (feeling of sleep), headache.
Common:may affect up to 1 in 10 people
- anorexia (loss of appetite);
- depression, hostility or aggression, anxiety, insomnia, nervousness or irritability;
- seizures, balance disorder, dizziness (feeling of instability), lethargy (lack of energy and enthusiasm), tremor (involuntary tremor);
- vertigo (feeling of rotation);
- cough;
- abdominal pain, diarrhea, dyspepsia (heavy digestion, heartburn, and acidity), vomiting, nausea;
- skin rash;
- asthenia/fatigue (feeling of weakness).
Uncommon:may affect up to 1 in 100 people
- decrease in platelet count, decrease in white blood cells;
- weight loss, weight gain;
- suicidal attempt and suicidal thoughts, mental disorders, abnormal behavior, hallucinations, anger, confusion, panic attack, emotional instability/mood changes, agitation;
- amnesia (memory loss), memory impairment (memory loss), abnormal coordination/ataxia (altered movement coordination), paresthesia (tingling), attention disorders (loss of concentration);
- diplopia (double vision), blurred vision;
- elevated/abnormal liver function test results;
- hair loss, eczema, itching;
- muscle weakness, myalgia (muscle pain);
- injury.
Rare:may affect up to 1 in 1,000 people
- infection;
- decrease in all types of blood cells;
- severe allergic reactions (DRESS, anaphylactic reaction [severe and important allergic reaction], Quincke's edema [swelling of face, lips, tongue, and throat]);
- decrease in sodium concentration in blood;
- suicide, personality disorders (behavioral problems), abnormal thinking (slow thinking, difficulty concentrating);
- delirium;
- encephalopathy (see subsection "Tell your doctor immediately" for a detailed description of symptoms);
- epileptic seizures may worsen or occur more frequently;
- uncontrolled muscle spasms affecting the head, torso, and limbs, difficulty controlling movements, hyperkinesia (hyperactivity);
- change in heart rhythm (electrocardiogram);
- pancreatitis (inflammation of the pancreas);
- liver failure, hepatitis (inflammation of the liver);
- sudden decreased renal function;
- skin rash, which can lead to blisters that may appear as small targets (dark central spots surrounded by a paler area, with a dark ring around the edge) (erythema multiforme), a widespread rash with blisters and skin peeling, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome), and a more severe form that causes skin peeling on more than 30% of the body surface (toxic epidermal necrolysis);
- rhabdomyolysis (muscle tissue breakdown) and increased creatine phosphokinase in blood. The prevalence is significantly higher in Japanese patients compared to non-Japanese patients;
- limping or difficulty walking;
- a combination of fever, muscle stiffness, unstable blood pressure and heart rate, confusion, low level of consciousness (may be signs of a disorder called malignant neuroleptic syndrome). The prevalence is significantly higher in Japanese patients compared to non-Japanese patients.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Levetiracetam ratiopharm
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging and on the bottle after EXP. The expiration date is the last day of the month indicated.
Store the bottle in the original packaging to protect it from light.
Do not use after 4 months of opening the package.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Levetiracetam ratiopharm
The active ingredient is levetiracetam. Each ml contains 100 mg of levetiracetam.
The other ingredients are: methyl parahydroxybenzoate (E128), propyl parahydroxybenzoate (E216), citric acid monohydrate, sodium hydroxide, purified water, acesulfame potassium (E950), grape flavor
Appearance of the Product and Package Contents
Levetiracetam ratiopharm oral solution is a clear liquid.
The 300 ml glass bottle of Levetiracetam ratiopharm oral solution (for children 4 years of age and older, adolescents, and adults) is packaged in a cardboard box containing a 10 ml syringe (graduated every 0.25 ml) and a syringe adapter.
The 150 ml glass bottle of Levetiracetam ratiopharm oral solution (for infants 6 months and older and children 2 to 4 years of age) is packaged in a cardboard box containing a 3 ml syringe (graduated every 0.1 ml) and a syringe adapter.
The 150 ml glass bottle of Levetiracetam ratiopharm oral solution (for infants 1 month and younger than 6 months of age) is packaged in a cardboard box containing a 1 ml syringe (graduated every 0.05 ml) and a syringe adapter.
Marketing Authorization Holder
ratiopharm GmbH
Graf-Arco-Strasse 3
89079 Ulm
Germany
Email: [email protected]
Manufacturer
Merckle GmbH
Ludwig-Merckle-Strasse 3
89143 Blaubeuren
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Teva Pharma Belgium N.V./S.A./AG Tel./Tél.: +32 38207373 | Lietuva UAB Teva Baltics Tel: +370 52660203 |
| Luxembourg/Luxemburg ratiopharm GmbH Allemagne/Deutschland Tél/Tel: +49 73140202 |
Ceská republika Teva Pharmaceuticals CR, s.r.o. Tel: +420 251007111 | Magyarország Teva Gyógyszergyár Zrt. Tel: +36 12886400 |
Danmark Teva Denmark A/S Tlf: +45 44985511 | Malta Teva Pharmaceuticals Ireland L-Irlanda Tel: +44 2075407117 |
Deutschland ratiopharm GmbH Tel: +49 73140202 | Nederland Teva Nederland B.V. Tel.: +31 8000228400 |
Eesti UAB Teva Baltics Eesti filiaal Tel: +372 6610801 | Norge Teva Norway AS Tlf: +47 66775590 |
Ελλáδα Specifar A.B.E.E. Τηλ: +30 2118805000 | Österreich ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
España Teva Pharma, S.L.U. Tél: +34 913873280 | Polska Teva Pharmaceuticals Polska Sp.z.o.o Tel: +48 223459300 |
France Teva Santé Tél: +33 155917800 | Portugal ratiopharm, Comércio e Indústria de Produtos Farmacêuticos Lda. Tel: +351 214767550 |
Hrvatska Pliva Hrvatska d.o.o. Tel: +385 13720000 | România Teva Pharmaceuticals S.R.L. Tel: +40 212306524 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 2075407117 | Slovenija Pliva Ljubljana d.o.o. Tel: +386 15890390 |
Ísland Teva Pharma Iceland ehf. Sími: +354 5503300 | Slovenská republika Teva Pharmaceuticals Slovakia s.r.o. Tel: +421 257267911 |
Italia Teva Italia S.r.l. Tel: +39 028917981 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 201805900 |
Κúπρος Specifar A.B.E.E. Ελλáδα Τηλ: +30 2118805000 | Sverige Teva Sweden AB Tel: +46 42121100 |
Latvija UAB Teva Baltics filiale Latvija Tel: +371 67323666 | United Kingdom (Northern Ireland) Teva Pharmaceuticals Ireland Ireland Tel: +44 2075407117 |
Date of Last Revision of this Leaflet:{MM/AAAA}
Detailed information on this medicine is available on the European Medicines Agency website http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEVETIRACETAM RATIOPHARM 100 mg/ml ORAL SOLUTIONDosage form: INJECTABLE PERFUSION, 100 mgActive substance: levetiracetamManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE PERFUSION, 100 mg/mlActive substance: levetiracetamManufacturer: Ucb PharmaPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 100 mgActive substance: levetiracetamManufacturer: Ucb PharmaPrescription required
Online doctors for LEVETIRACETAM RATIOPHARM 100 mg/ml ORAL SOLUTION
Discuss questions about LEVETIRACETAM RATIOPHARM 100 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions









