
LENZETTO 1.53 mg/dose TRANSDERMAL SPRAY SOLUTION


How to use LENZETTO 1.53 mg/dose TRANSDERMAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Lenzetto 1.53 mg/dose, solution for transdermal spray
estradiol
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again. If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Lenzetto and what is it used for
- What you need to know before starting to use Lenzetto
- How to use Lenzetto
- Possible side effects
- Storage of Lenzetto
- Package contents and additional information
1. What is Lenzetto and what is it used for
Lenzetto is a Hormone Replacement Therapy (HRT). It contains the female hormone estrogen. Lenzetto is used in postmenopausal women when at least 6 months have passed since their last natural menstrual period.
Lenzetto can also be used in women who have had surgery to remove their ovaries, as this causes instant menopause.
Lenzetto is a spray solution that contains small amounts of a medication called estradiol. When sprayed onto the skin as directed, it passes through the skin into the bloodstream.
Lenzetto is used to:
Relief of symptoms that occur after menopause
During menopause, the amount of estrogen produced by the woman's body decreases. This can cause symptoms such as heat in the face, neck, and chest ("hot flashes"). Lenzetto relieves these symptoms after menopause. Lenzetto will only be prescribed to you if your symptoms seriously affect your daily life.
Lenzetto is indicated for the treatment of estrogen deficiency symptoms after menopause, when menstruation no longer occurs after menopause. Estrogen deficiency symptoms include hot flashes (sudden waves of heat and sweating all over the body), sleep problems, irritability, and vaginal dryness.
Experience in treating women over 65 years of age is limited.
Lenzetto is not a contraceptive.
2. What you need to know before starting to use Lenzetto
Medical history and regular check-ups:
The use of HRT carries risks that need to be considered when deciding whether to start using it, or whether to continue using it.
Experience in the treatment of women with premature menopause (due to ovarian failure or surgery) is limited. If you have premature menopause, the risks of using HRT may be different. Please consult your doctor.
Before starting (or resuming) HRT, your doctor will ask you about your personal and family medical history. Your doctor may decide to perform a physical examination. This may include an examination of your breasts and/or an internal examination, if necessary.
Once you have started treatment with Lenzetto, you should visit your doctor for regular check-ups (at least once a year). During these check-ups, discuss the benefits and risks of continuing with Lenzetto with your doctor.
Regularly undergo breast examinations as recommended by your doctor.
Do not use Lenzetto
If any of the following situations apply to you. If you are unsure about any of the following points, consult your doctorbefore using Lenzetto.
Do not use Lenzetto
- if you have or have had breast cancer, or if you suspect you have it;
- if you have estrogen-dependent cancer, such as cancer of the lining of the uterus (endometrium), or if you suspect you have it;
- if you have any unexplained vaginal bleeding;
- if you have excessive thickening of the lining of the uterus(endometrial hyperplasia) that is not being treated;
- if you have or have had a blood clot in a vein(thrombosis), such as in the legs (deep vein thrombosis) or in the lungs (pulmonary embolism);
- if you have a blood clotting disorder(such as protein C, protein S, or antithrombin deficiency);
- if you have or have recently had a disease caused by blood clots in the arteries, such as a heart attack, stroke, or angina;
- if you have, or have ever had, a liver diseaseand your liver function tests have not returned to normal;
- if you have a rare blood disease called "porphyria" that is inherited in families (hereditary);
- if you are allergicto estradiol or any of the other components of Lenzetto (listed in section 6).
If any of the above conditions appear for the first time during the use of this medicine, stop treatment immediately and consult your doctor immediately.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Lenzetto.
Tell your doctor if you have ever had any of the following problems, before starting treatment, as they may appear again or worsen during treatment with Lenzetto. In this case, you should visit your doctor more frequently for check-ups:
- fibroids in your uterus;
- growth of the lining of the uterus outside the uterus (endometriosis) or a history of excessive growth of the lining of the uterus (endometrial hyperplasia);
- increased risk of developing blood clots (see "Blood clot in a vein (thrombosis)");
- increased risk of developing estrogen-dependent cancer (such as having a mother, sister, or grandmother who has had breast cancer);
- high blood pressure;
- a liver disorder, such as a benign tumor in the liver;
- diabetes;
- gallstones;
- migraine or severe headache;
- a disease of the immune system that affects several organs of the body (Systemic Lupus Erythematosus, SLE);
- epilepsy;
- asthma;
- a disease that affects the eardrum and ear (otosclerosis);
- a very high level of fat in your blood (triglycerides);
- fluid retention due to heart or kidney problems;
- hereditary and acquired angioedema.
Stop treatment with Lenzetto and go to a doctor immediately
If you notice any of the following symptoms while using HRT:
- any of the situations mentioned in the section "Do not use Lenzetto";
- yellowing of your skin or the whites of your eyes (jaundice). These can be signs of liver disease;
- swelling of the face, tongue, or throat, and difficulty swallowing or urticaria accompanied by difficulty breathing, which suggest angioedema;
- a significant increase in your blood pressure (symptoms may be headache, fatigue, dizziness);
- migraine-like headaches that occur for the first time;
- if you become pregnant;
- if you notice signs of a blood clot, such as:
- painful swelling and redness of the legs
- sudden chest pain
- difficulty breathing.
For more information, see "Blood clot in a vein (thrombosis)".
Note: Lenzetto is not a contraceptive. If it has been less than 12 months since your last menstrual period or you are under 50 years old, you may still need additional contraceptive measures to prevent pregnancy. Ask your doctor for advice.
HRT and cancer
Excessive thickening of the lining of the uterus (endometrial hyperplasia) and cancer of the lining of the uterus (endometrial cancer).
HRT with only estrogen will increase the risk of excessive thickening of the lining of the uterus (endometrial hyperplasia) and cancer of the lining of the uterus (endometrial cancer).
Taking a progestogen in addition to estrogen for at least 12 days of each 28-day cycle will protect you from this additional risk. Therefore, your doctor will prescribe a progestogen separately if you still have a uterus. If you have had a hysterectomy, consult your doctor to see if you can use this medicine safely without a progestogen.
In women with a uterus who are not treated with HRT, on average, 5 out of 1,000 will be diagnosed with endometrial cancer between the ages of 50 and 65.
In women between the ages of 50 and 65 who are treated with HRT with only estrogens, between 10 and 60 women out of 1,000 will be diagnosed with endometrial cancer (i.e., between 5 and 55 additional cases), depending on the dose and duration of treatment.
Lenzetto contains a higher dose of estrogen than other HRT products with only estrogens. The risk of endometrial cancer when using Lenzetto with a progestogen is unknown.
Unexpected bleeding
You will have a bleed once a month (called withdrawal bleeding) while using Lenzetto if it is combined with a sequential progestogen. But if you have unexpected bleeding or spotting in addition to your monthly bleed, which:
- continues after the first 6 months;
- starts after you have been using Lenzetto for more than 6 months;
- continues after you have stopped using Lenzetto;
go to your doctor as soon as possible.
Breast cancer
It has been shown that combined estrogen-progestogen HRT or HRT with only estrogens increases the risk of breast cancer. The additional risk depends on the duration of HRT use. The additional risk becomes apparent within 3 years of use. After stopping HRT, the additional risk will decrease over time, but may persist for 10 years or more if HRT has been used for more than 5 years.
Comparison
In women aged 50-54 who do not use HRT, on average, 13-17 out of 1,000 will be diagnosed with breast cancer over a 5-year period.
In women aged 50 who start using HRT with only estrogens for 5 years, there will be 16-17 cases per 1,000 users (i.e., 0-3 additional cases).
In women aged 50 who start using combined estrogen-progestogen HRT for 5 years, there will be 21 cases per 1,000 users (i.e., 4-8 additional cases).
In women aged 50-59 who do not use HRT, breast cancer will be diagnosed in an average of 27 out of 1,000 over a 10-year period.
In women aged 50 who start using HRT with only estrogens for 10 years, there will be 34 cases per 1,000 users (i.e., 7 additional cases).
In women aged 50 who start using combined estrogen-progestogen HRT for 10 years, there will be 48 cases per 1,000 users (i.e., 21 additional cases).
Examine your breasts regularly. Go to your doctor if you notice any changes, such as:
- formation of dimples in the skin
- changes in the nipple
- any lump that you can see or feel.
In addition, you are advised to participate in breast screening programs when they are offered to you. For mammography, it is important to inform the nurse/healthcare professional who will perform the X-ray that you are using HRT, as this medicine may increase the density of your breasts, which can affect the result of the mammography. When breast density increases, it is possible that mammography may not detect all lumps.
Ovarian cancer
Ovarian cancer is rare, much less common than breast cancer. The use of HRT with estrogens alone or in combination with estrogens-progestogens has been associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer varies with age. For example, in women aged 50-54 who do not use HRT, there are approximately 2 cases of ovarian cancer per 2,000 women over a 5-year period. In women treated with HRT for 5 years, there are approximately 3 cases per 2,000 patients (i.e., approximately 1 additional case).
Effect of HRT on the heart and circulation
Blood clots in a vein (thrombosis)
The risk of blood clots in the veinsis approximately 1.3 to 3 times higher in HRT users than in non-users, especially during the first year of treatment.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, difficulty breathing, fainting, or even death.
You are more likely to develop a blood clot in your veins as you get older and if you have any of the following conditions. Inform your doctor if you have any of the following conditions:
- you are unable to walk for a long time due to major surgery, injury, or illness (see also section 3, "If you need surgery").
- you are severely overweight (BMI >30 kg/m2).
- you have a blood clotting disorder that requires long-term treatment with a medicine used to prevent blood clots.
- if any of your close relatives have ever had a blood clot in a leg, lung, or other organ.
- you have systemic lupus erythematosus (SLE).
- you have cancer.
For signs of a blood clot, see "Stop treatment with Lenzetto and go to a doctor immediately".
Considering women aged 50 who do not use HRT, on average, over a 5-year period, 4-7 out of 1,000 will have a blood clot in a vein.
In women aged 50 who have been using combined estrogen-progestogen HRT for 5 years, there will be 9-12 cases per 1,000 users (i.e., 5 additional cases).
In women aged 50 who have had a hysterectomy and have been using HRT with only estrogens for 5 years, there will be 5-8 cases per 1,000 users (i.e., 1 additional case).
Heart disease (heart attack)
There is no evidence that HRT prevents a heart attack.
Women over 60 who use combined estrogen-progestogen HRT are slightly more likely to develop heart disease than those who do not use HRT.
In the case of women who have had a hysterectomy and only use estrogen therapy, there is no increased risk of developing heart disease.
Stroke
The risk of having a stroke is approximately 1.5 times higher in women treated with HRT than in those not treated. The number of additional stroke cases due to HRT use will increase with age.
Comparison: In women aged 50 who do not use HRT, on average, 8 out of 1,000 will have a stroke over a 5-year period. In women aged 50 who use HRT, there will be 11 cases per 1,000 users over 5 years (i.e., 3 additional cases).
Other conditions
HRT does not prevent memory loss. There is some evidence of an increased risk of memory loss in women who start using HRT after the age of 65. Consult your doctor.
Children
The estradiol spray can be accidentally transferred from the skin where it has been sprayed to other people. Do not allow others, especially children, to come into contact with the exposed area of your skin, and cover the area if necessary, after the spray has dried. If a child comes into contact with the area of skin where Lenzetto has been sprayed, wash the child's skin with soap and water as soon as possible. Due to the transfer of estradiol, small children may show signs of unexpected puberty (e.g., breast development). In most cases, the symptoms will disappear when the children stop being exposed to the estradiol spray.
Contact your doctor if you notice any signs or symptoms (breast development or other sexual changes) in a child who may have been accidentally exposed to the estradiol spray.
Other medicines and Lenzetto
Tell your doctor if you are using, have recently used, or might use any other medicines.
Some medicines may interfere with the effect of Lenzetto. This may cause irregular bleeding. This occurs with the following medicines:
- Medicines for epilepsy(such as phenobarbital, phenytoin, and carbamazepine)
- Medicines for tuberculosis(such as rifampicin, rifabutin)
- Medicines for HIV infection(such as nevirapine, efavirenz, ritonavir, and nelfinavir)
- Medicines (herbal) that contain St. John's Wort(Hypericum perforatum)
HRT may affect the way other medicines work:
- A medicine for epilepsy (lamotrigine), as it may increase the frequency of seizures;
- Medicines for hepatitis C virus (HCV) (e.g., combination regimen for HCV ombitasvir/paritaprevir/ritonavir with or without dasabuvir or glecaprevir/pibrentasvir) may cause elevations in blood test results for liver function (increase in liver enzyme ALT) in women using HRT with ethinylestradiol. Lenzetto contains estradiol instead of ethinylestradiol. It is not known whether an increase in liver enzyme ALT can occur when using Lenzetto with this combination regimen for HCV.
Tell your doctor or pharmacist if you are using or have recently used other medicines, including medicines without a prescription, herbal medicines, or other natural products.
Laboratory tests
If you need a blood test, tell your doctor or laboratory staff that you are using Lenzetto, as this medicine may affect the results of some tests.
Pregnancy and breastfeeding
Lenzetto is for use only in postmenopausal women. If you become pregnant, stop treatment with Lenzetto and contact your doctor.
Do not use Lenzetto while breastfeeding.
Driving and using machines
Lenzetto has no known effects on the ability to drive or use machines.
Lenzetto contains alcohol
This medicine contains 65.47 mg of alcohol (ethanol) per dose, which is equivalent to 72.74% p/v. It may cause a burning sensation on damaged skin.
Alcohol-based products are flammable. Keep away from fire. Avoid open flames, lit cigarettes, or the use of appliances that are a source of heat (e.g., hair dryers) while applying the spray to your skin, until the spray has dried.
3. How to use Lenzetto
Follow your doctor's instructions for administering this medication exactly. If in doubt, consult your doctor or pharmacist again.
Your doctor will prescribe the lowest dose to treat your symptoms for the necessary time.
During treatment, your doctor may adjust the dose according to your individual needs. Talk to your doctor if you think the dose is too strong or insufficient.
If you have not undergone a hysterectomy (surgical intervention to remove the uterus), your doctor will give you tablets containing another hormone called progestogen to counteract the effects of estrogen on the uterine lining. Your doctor will explain how to take these tablets. At the end of the treatment period with progestogens, a withdrawal bleed (see section "unexpected bleeding") may occur.
If you need surgery
If you are going to undergo surgery, inform the surgeon that you are using Lenzetto. You may need to stop using Lenzetto around 4 to 6 weeks before the operation to reduce the risk of a blood clot (see section 2, Blood clots in a vein). Ask your doctor when you can start using Lenzetto again.
Where to apply Lenzetto
The spray should be applied to dry, healthy skin on the inside of the forearm. If this is not possible, it should be applied to the inside of the thigh.
Do not apply Lenzetto to the breasts or any area near the breasts.
How to apply Lenzetto
Before using a new applicator for the first time, the pump must be primed by spraying three times with the cap on:The container should be kept in a vertical position as shown in Figure 1. With the cap on, press the button down three times with your thumb or index finger.
The medication is now ready for use.
DO NOT prepare the applicator before each dose; prepare it only once before starting to use a new container. If you forgot one or more doses, prepare the applicator according to the instructions in the section “If you forgot to use Lenzetto”.
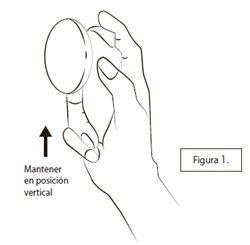
Make sure the skin where you want to spray the medication is healthy, clean, and dry.
How to apply your daily dose.
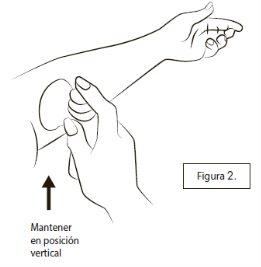
To apply your daily dose, remove the plastic cap, hold the container in a vertical position, and place the plastic cone flat against the skin (Figure 2).
You may need to move your arm or move the cone over your arm so that the cone is flat against the skin and there are no gaps between the cone and the skin.
Press the button down once. It should be pressed all the way down and held before releasing.
If you need another spray, move the cone along your arm so that it is next to the area you have already sprayed. Press the button down once.
If you need a third spray, move the cone along your arm again and press the button down once.
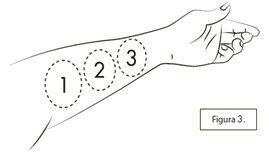
If the second or third spray does not fit on the same inner forearm, you can also spray on the inner thigh. If you have trouble putting the cone on the inner forearm as shown in Figure 3, or if you find it difficult to use it on your forearms, you can also spray on the inner surface of your thigh.
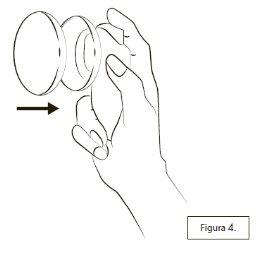
When you have finished using Lenzetto, always put the cap on the container (Figure 4).
If the medication is used according to the instructions, regardless of the different forms or pattern of administration on the skin, each spray will release the same amount of active ingredient.
Let the spray dry for at least 2minutes before dressing and at least 60minutes before bathing or washing. If Lenzetto spray comes into contact with another area of skin such as the hands, wash that area of skin with water and soap immediately.
Lenzetto cannot be used on damaged or broken skin.
Do not massage or rub Lenzetto into the skin.
Do not allow others to touch the area of skin where the spray has been applied until it has dried and cover it with clothing 2 minutes after application, if necessary.If another person (especially a child) accidentally touches the area of your skin where you have sprayed Lenzetto, tell them to wash the area of their skin with water and soap immediately.
How much Lenzetto to use
Your doctor will likely recommend the lowest dose initially (one spray per day) and you should talk to your doctor about how the medication is working for you. If necessary, your doctor may increase the dose to two sprays per day. The maximum daily dose is 3 sprays.
How often to use Lenzetto
You should apply the total number of sprays (dose) that your doctor has prescribed at the same time every day.
How long to use Lenzetto
Talk to your doctor every 3-6 months about how long you should use Lenzetto. You should only use Lenzetto for as long as you need it to relieve hot flashes associated with menopause.
Other useful information
Sunscreens may alter the absorption of Lenzetto's estrogen.
Avoid using sunscreen on the area of skin where you plan to spray. However, if you need to use sunscreen, you should apply it at least one hour before using Lenzetto.
Lenzetto should be used with caution in extreme temperature conditions, such as in a sauna or when sunbathing.
There is limited data suggesting that the speed and degree of absorption of Lenzetto may be reduced in women with overweight and obesity. Consult your doctor. During treatment, your doctor may adjust the dose according to your individual needs.
If you use more Lenzetto than you should
If you use more Lenzetto than you should, or if children have been using the medication by accident, contact your doctor or hospital for advice on the risk and measures to be taken.
If you use more Lenzetto than you should, you may feel unwell, vomit, and have a withdrawal bleed (unusual vaginal bleeding).
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount used.
If you forgot to use Lenzetto
If you forgot to use Lenzetto at your usual time, apply the medication as soon as you remember and, the next day, use it as you normally would. If it is almost time for your next dose, wait and apply the next dose as you normally would. If you forget one or more doses, a first spray with the protective cap on will be necessary. Do not use a double dose to make up for forgotten doses.
Forgetting a dose may increase the likelihood of intermenstrual bleeding and spotting.
If you have any other questions about using this medication, ask your doctor or pharmacist.
If you stop treatment with Lenzetto
Your doctor will explain how to stop treatment with this medication when your treatment is finished.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The following diseases have been reported more frequently in women using HRT compared to women not using HRT:
- breast cancer;
- abnormal growth or cancer of the inner lining of the uterus (hyperplasia or endometrial cancer);
- ovarian cancer;
- blood clots in the veins of the legs or lungs (venous thromboembolism);
- heart disease;
- stroke;
- gallbladder disease;
- high blood pressure;
- liver problems;
- high blood sugar levels;
- possible memory loss if HRT is started after age 65.
For more information on these side effects, see section 2.
Some side effects can be serious
The following symptoms require immediate medical attention:
- sudden chest pain;
- chest pain that spreads to the arm or neck;
- difficulty breathing;
- painful swelling and redness in the legs;
- yellowing of the eyes and face (jaundice);
- unusual vaginal bleeding (intermenstrual bleeding) or spotting after using Lenzetto for a while or after stopping treatment;
- changes in the breasts, including dimpling of the skin, changes in the nipple, lumps that can be seen or felt;
- painful periods;
- dizziness and fainting;
- changes in speech;
- changes in vision;
- migraine-like headaches without a known cause.
If any of the side effects you suffer from worsen or if you notice any side effect not mentioned in this leaflet, tell your doctor or pharmacist.
The following side effects have been reported with Lenzetto:
Common side effects(may affect up to 1 in 10 people)
Headache, abdominal pain, nausea, rash, itching, irregular uterine bleeding or vaginal bleeding including spotting, breast tenderness, breast pain, weight gain or loss.
Uncommon side effects(may affect up to 1 in 100 people)
Hypersensitivity reactions, depressive mood, insomnia, dizziness, vertigo, visual disturbances, palpitations, diarrhea, dyspepsia, increased blood pressure, erythema nodosum, urticaria, skin irritation, edema, muscle pain, breast discoloration, breast secretion, uterine or cervical polyps, endometrial hyperplasia, ovarian cyst, vaginal inflammation, vaginal candidiasis, elevated liver enzymes and cholesterol levels, armpit pain.
Rare side effects(may affect up to 1 in 1,000 people)
Anxiety, increased or decreased sexual desire, migraine, intolerance to contact lenses, abdominal swelling, vomiting, increased body hair, acne, muscle cramps, painful menstruation, premenstrual syndrome, breast enlargement, fatigue.
Other side effectshave been reported with a frequency "not known" (frequency cannot be estimated from available data) with Lenzetto during post-marketing surveillance: hair loss (alopecia), chloasma (brownish-yellow patches, especially on the face), skin discoloration.
The following side effects have been reported with other HRTs
Severe allergic reaction that causes swelling of the face or throat (angioedema), anaphylactoid/anaphylactic reactions (severe allergic reaction that causes difficulty breathing or dizziness), glucose intolerance, depression, mood changes, irritability, exacerbation of chorea, exacerbation of epilepsy, dementia, exacerbation of asthma, gallbladder disease, yellowing of the skin (jaundice), pancreatitis, benign tumor of the smooth muscle of the uterus, various skin disorders: skin discoloration, especially on the face or neck, known as "pregnancy patches" (chloasma); red, painful skin nodules (erythema nodosum); rash with redness in a target shape or sores (erythema multiforme), hemorrhagic rash, hair loss, joint pain, breast secretion, breast lumps, increased size of uterine or cervical polyps, changes in cervical secretion and lining, vaginal inflammation, vaginal candidiasis, low calcium levels in the blood.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medicines Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Lenzetto
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the box and label after EXP. The expiration date is the last day of the month indicated.
Use within 56 days of first use.
Do not refrigerate or freeze this medication.
Do not store at a temperature above 25°C.
Contains ethanol, which is flammable. Keep away from heaters, open flames, and other ignition sources.
Medications should not be disposed of in wastewater or household waste. Place the containers and medications you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Lenzetto
- The active ingredient is estradiol (as estradiol hemihydrate). Each spray contains 1.53 mg of estradiol (equivalent to 1.58 mg of estradiol hemihydrate).
- The other ingredients are octisalate and ethanol 96%.
Appearance and package contents
Lenzetto is a transdermal spray that contains a solution of estradiol and octisalate in ethanol. It comes with a dosing pump.
Lenzetto is presented in a container with a plastic cap. Inside, there is a glass container that contains 6.5 ml of solution and is designed to deliver 56 sprays of 90 microliters after priming the dosing pump. Mark each spray made on the box table.
Each spray contains 1.53 mg of estradiol.
Use only the number of sprays indicated on the label of each Lenzetto container, even if the container is not completely empty.
Container sizes:
1 container of 6.5 ml (56 sprays).
3 containers of 3 x 6.5 ml (3 x 56 sprays).
Not all container sizes may be marketed.
Marketing authorization holder
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungary
Manufacturer
Gedeon Richter România S.A.
Cuza Voda Street 99-105
Târgu-Mures
Romania - 540306
or
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungary
You can request more information about this medication from the local representative of the marketing authorization holder:
Gedeon Richter Ibérica S.A.
Sabino Arana nº 28, 4º 2ª
08028 Barcelona, Spain
Date of last revision of this leaflet:January 2024
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.04 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LENZETTO 1.53 mg/dose TRANSDERMAL SPRAY SOLUTIONDosage form: GEL, 0.5 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: GEL, 1 mgActive substance: estradiolManufacturer: Orion CorporationPrescription requiredDosage form: TRANSDERMAL PATCH, 3 mgActive substance: estradiolManufacturer: Merus Labs Luxco Ii S.À.R.L.Prescription required
Online doctors for LENZETTO 1.53 mg/dose TRANSDERMAL SPRAY SOLUTION
Discuss questions about LENZETTO 1.53 mg/dose TRANSDERMAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











