
LEMTRADA 12 mg concentrate for infusion solution

How to use LEMTRADA 12 mg concentrate for infusion solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
LEMTRADA12mg concentrate for solution for infusion
alemtuzumab
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may have. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you are given this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you experience any side effects, talk to your doctor, or pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is LEMTRADA and what is it used for
- What you need to know before you are given LEMTRADA
- How LEMTRADA will be given
- Possible side effects
- Storing LEMTRADA
- Contents of the pack and other information
1. What is LEMTRADA and what is it used for
LEMTRADA contains the active substance alemtuzumab, which is used to treat a form of multiple sclerosis (MS) in adults, called relapsing-remitting multiple sclerosis (RRMS). LEMTRADA does not cure MS, but it can reduce the number of relapses. It may also slow down or reverse some of the signs and symptoms of MS. In clinical trials, patients treated with LEMTRADA had fewer relapses and were less likely to experience worsening of their disability compared to patients treated with an interferon beta injected several times a week.
LEMTRADA is used if your MS is highly active despite having been treated with at least one other MS medicine, or if your MS is quickly getting worse.
What is multiple sclerosis?
MS is an autoimmune disease that affects the central nervous system (brain and spinal cord). In MS, the immune system mistakenly attacks the protective covering (myelin) that surrounds nerve fibers, causing inflammation. When this inflammation causes symptoms, it is usually called a "relapse" or "attack". In RRMS, patients experience relapses followed by periods of recovery.
The symptoms you experience depend on which part of the central nervous system is affected. The damage to the nerves during this inflammation can be reversible, but as the disease progresses, the damage can accumulate and become permanent.
HowLEMTRADA works
LEMTRADA regulates the immune system to limit its attacks on the nervous system.
2. What you need to know before you are given LEMTRADA
DO NOT use LEMTRADA:
- if you are allergic to alemtuzumab or to any of the other ingredients of this medicine (listed in section 6)
- if you are a carrier of the human immunodeficiency virus (HIV)
- if you have a severe infection
- if you have any of the following diseases:
- any other autoimmune disease in addition to multiple sclerosis
- uncontrolled high blood pressure
- a history of tears in the blood vessels that supply the brain
- a history of stroke (cerebrovascular accident)
- a history of heart attack or chest pain
- a history of bleeding disorder.
Warnings and precautions
Consult your doctor before you are given LEMTRADA. After completing a treatment cycle with LEMTRADA, you may have a higher risk of developing other autoimmune diseases or experiencing severe infections. It is essential that you understand these risks and know how to detect them. You will be provided with a Patient Card and a Patient Guide with more information. It is crucial that you carry the Patient Card with you during treatment and up to 4 years after the last infusion of LEMTRADA, as side effects can occur years after treatment. When you are under medical care, even if it is not for MS, show the Patient Card to your doctor.
Your doctor will perform blood tests before starting treatment with LEMTRADA. These tests are done to determine if you can take LEMTRADA. Your doctor will also ensure that you do not have certain diseases or medical conditions before starting treatment with LEMTRADA.
- Autoimmune diseases
Treatment with LEMTRADA may increase the risk of autoimmune diseases. These diseases are characterized by the immune system attacking the body by mistake. The following information is provided about specific diseases that have been observed in MS patients treated with LEMTRADA.
Autoimmune diseases can occur many years after treatment with LEMTRADA. Therefore, it is necessary to perform blood and urine tests up to 4 years after the last infusion. The tests are necessary, even if you feel well and your MS symptoms are under control. There are certain signs and symptoms that you should watch out for yourself. Additionally, these diseases can appear beyond 4 years, so you must be alert to the signs and symptoms, even after it is no longer necessary to perform monthly blood and urine tests. In sections 2 and 4 - autoimmune diseases, details are provided about the signs and symptoms, tests, and actions you should take.
The LEMTRADA patient guideprovides more useful information about these autoimmune diseases (and associated tests).
- Acquired hemophilia A
Rarely, patients developed a bleeding disordercaused by antibodies that act against factor VIII (a protein necessary for normal blood clotting), called acquired hemophilia A. This disease must be diagnosed and treated immediately. The symptoms of acquired hemophilia A are described in section 4.
- Immune thrombocytopenic purpura (ITP)
Frequently, patients have developed a bleeding disordercaused by a low platelet count, called immune thrombocytopenic purpura (ITP). This disorder must be detected and treated quickly, as otherwise, its effects can be severe or even fatal. The signs and symptoms of ITP are described in section 4.
- Kidney disease (such as anti-MBG disease)
Rarely, patients have experienced problems related to autoimmune disorders in the kidneys, such as anti-membranous glomerular disease (anti-MBG disease). The signs and symptoms of kidney disease are described in section 4. If left untreated, it can lead to kidney failure requiring dialysis or transplantation and could be fatal.
- Thyroid disorders
Very frequently, patients have experienced an autoimmune disorder of the thyroid glandthat affects its ability to produce or control important hormones for metabolism.
LEMTRADA can cause different types of thyroid disorders, including:
- Overactive thyroid gland(hyperthyroidism) when the thyroid produces too much hormone
- Underactive thyroid gland(hypothyroidism) when the thyroid does not produce enough hormone
The signs and symptoms of thyroid disorders are described in section 4.
If you develop a thyroid disorder, in most cases, you will need treatment for the rest of your life with medications that control the disorder, and in some cases, it may be necessary to remove the thyroid gland.
It is very important to follow the appropriate treatment for the thyroid disorder, especially if you become pregnant after using LEMTRADA. An untreated thyroid disorder could harm the fetus or baby after birth.
- Liver inflammation
Some patients have developed liver inflammation after receiving LEMTRADA. Liver inflammation can be diagnosed from blood tests that will be performed regularly after treatment with LEMTRADA. Inform your doctor if you experience one or more of the following symptoms: nausea, vomiting, abdominal pain, fatigue, loss of appetite, yellow skin or eyes, dark urine, or bleeding or bruising more easily than usual.
- Thrombotic thrombocytopenic purpura (TTP)
With LEMTRADA, a blood clotting disorder called thrombotic thrombocytopenic purpura (TTP) can occur. Blood clots form in blood vessels and can occur throughout the body. Seek immediate medical help if you experience any of the following symptoms: skin or mouth bruises that can appear as small red dots, with or without extreme unexplained fatigue, fever, confusion, speech changes, yellowing of the skin or eyes (jaundice), low urine output, or dark urine. It is recommended to seek urgent medical attention, as prolonged TTP can be fatal (see section 4 "Possible side effects").
- Sarcoidosis
A disorder of the immune system (sarcoidosis) has been reported in patients treated with LEMTRADA. Symptoms may include persistent dry cough, shortness of breath, chest pain, fever, swollen lymph nodes, weight loss, skin rashes, and blurred vision.
- Autoimmune encephalitis
Autoimmune encephalitis (an immune-mediated brain disorder) can occur after receiving LEMTRADA. This condition can include symptoms such as behavioral and/or psychiatric changes, short-term memory loss, or seizures. The symptoms can resemble an MS relapse. If you develop one or more of these symptoms, contact your doctor.
- Other autoimmune diseases
Rarely, some patients have experienced autoimmune diseases related to red blood cells or white blood cells. These diseases can be diagnosed from blood tests that will be performed regularly after treatment with LEMTRADA. If you develop one of these diseases, your doctor will inform you and take appropriate measures to treat it.
- Infusion reactions
Most patients treated with LEMTRADA will experience side effects at the time of infusion or within 24 hours. To try to reduce infusion reactions, your doctor will treat you with other medications (see section 4 - infusion reactions).
- Other severe reactions occur shortly after LEMTRADA infusion
Some patients have had severe or potentially life-threatening reactions after infusion with LEMTRADA, including bleeding in the lung, heart attack, stroke, or tears in the blood vessels that supply the brain. Reactions can occur after any of the doses during the treatment cycle. In most cases, reactions occurred within days 1-3 of infusion. Your doctor will monitor your vital signs, including blood pressure, before and during infusion. Seek immediate help if you experience any of the following symptoms: difficulty breathing, coughing up blood, chest pain, facial drooping, sudden severe headache, weakness on one side of the body, difficulty speaking, or neck pain.
- Hemophagocytic lymphohistiocytosis
Treatment with LEMTRADA may increase the risk of excessive activation of white blood cells associated with inflammation (hemophagocytic lymphohistiocytosis), which can be fatal if not diagnosed and treated in time. If you experience multiple symptoms such as fever, swollen glands, bruising, or skin rash, contact your doctor immediately.
- Adult-onset Still's disease (AOSD)
AOSD is a rare condition that can cause inflammation in multiple organs with various symptoms such as fever >39 °C or 102.2 °F that lasts more than 1 week, pain, stiffness with or without inflammation in multiple joints, and/or skin rash. If you experience a combination of these symptoms, contact your doctor immediately.
- Infections
Patients treated with LEMTRADA have a higher risk of suffering from a severe infection(see section 4 - infections). In general, infections can be treated with standard medications.
To reduce the possibility of suffering from an infection, your doctor will check if the other medications you are taking may be affecting your immune system. For this reason, it is essential to inform your doctor of all medications you are taking.
Also, inform your doctor if you have a severe infection before starting treatment with LEMTRADA, as your doctor must delay treatment until the infection is resolved.
Patients treated with LEMTRADA have a higher risk of developing a herpes infection(e.g., mouth ulcers). In general, once a patient has had a herpes infection, they have a higher risk of developing another. It is also possible to develop a herpes infection for the first time. It is recommended that your doctor prescribe appropriate treatment to reduce the chances of developing a herpes infection, which should be taken during the days when LEMTRADA treatment is administered and during the month following treatment.
Additionally, infections can occur that can cause cervical abnormalities. Therefore, it is recommended that all patients undergo an annual review, such as a cervical smear. Your doctor will indicate what tests you need .
Infections caused by a virus called cytomegalovirushave been reported in patients treated with LEMTRADA. Most cases occurred within two months after administration of alemtuzumab. Inform your doctor immediately if you have symptoms of infection, such as fever or swollen glands.
Patients treated with LEMTRADA have had infections caused by a virus called Epstein-Barr virus (EBV), including cases with severe and sometimes fatal liver inflammation. Inform your doctor immediately if you have symptoms of infection, such as fever, swollen glands, or fatigue.
Patients treated with LEMTRADA also have a high risk of developing listeria infection(a bacterial infection caused by consuming contaminated food) .Listeria infection can cause severe illness, including meningitis, but can be treated with appropriate medications .To reduce this risk, it is recommended to avoid eating raw or undercooked meat, fresh cheese, and unpasteurized dairy products two weeks before treatment, during treatment, and for at least 1 month after treatment with LEMTRADA.
If you live in an area where tuberculosisis common, you may have a higher risk of contracting it. Your doctor will schedule tests to detect tuberculosis.
If you are a carrier of the hepatitis B or C virus(which affect the liver), it is necessary to take greater care before receiving LEMTRADA, as it is unknown whether treatment can lead to the activation of the infection, which could subsequently damage the liver.
There have been cases of a rare brain infection called progressive multifocal leukoencephalopathy (PML) in patients who received Lemtrada. PML has been reported in patients with other risk factors, specifically previous treatment with MS medications associated with PML.
PML can lead to severe disability over weeks or months and can be fatal.
Symptoms can be similar to an MS relapse and include progressive weakness or clumsy movements of the limbs, visual disturbances, speech difficulties, or changes in thinking, memory, and orientation, which can cause confusion and personality changes. It is essential to inform your family or caregivers about your treatment, as they may notice symptoms that you are not aware of. Inform your doctor immediately if you experience any symptoms suggestive of PML.
- Pneumonitis and pericarditis
In patients treated with LEMTRADA, pneumonitis (inflammation of lung tissue) has been reported. Most cases occurred in the first month after treatment with LEMTRADA. Cases of pericardial effusion (fluid accumulation around the heart) and pericarditis (inflammation of the membrane around the heart) have also been reported in patients treated with LEMTRADA. You should inform your doctor of symptoms such as difficulty breathing, cough, wheezing, chest pain or tightness, bleeding when coughing, as these symptoms can be caused by pneumonitis, pericardial effusion, or pericarditis.
- Gallbladder inflammation
LEMTRADA may increase the possibility of gallbladder inflammation. This can be a severe medical condition that can be potentially fatal. You should inform your doctor if you experience symptoms such as stomach pain or discomfort, fever, nausea, or vomiting.
- Previous cancer diagnosis
If you were diagnosed with cancer in the past, inform your doctor.
- Vaccines
It is unknown whether LEMTRADA affects the response to vaccines. If you have not completed the necessary standard vaccinations, your doctor will assess whether you should receive them before treatment with LEMTRADA. In particular, your doctor will consider vaccinating you against chickenpox if you have not had it. Any vaccination should be administered at least 6 weeks before starting a treatment cycle with LEMTRADA.
YOU MUST NOT receive certain types of vaccines (live virus vaccines) if you have recently received LEMTRADA.
Children and adolescents
LEMTRADA should not be used in children and adolescents under 18 years of age, as it has not been studied in MS patients under this age.
Other medications and LEMTRADA
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication (including vaccinations or herbal medicines).
Aside from LEMTRADA, there are other treatments (including those for MS or to treat other diseases) that can affect the immune system and, therefore, affect your ability to fight infections. If you are using any of these medications, your doctor may ask you to stop taking them before starting treatment with LEMTRADA.
Pregnancy
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before you are given this medicine.
Women of childbearing age must use effective contraceptive methods during each treatment cycle with LEMTRADA and for 4 months after each cycle.
Special care should be taken if you become pregnant after treatment with LEMTRADA and experience a thyroid disorder during pregnancy. Thyroid disorders can harm the baby (see section 2 Warnings and precautions - autoimmune diseases).
Breastfeeding
It is unknown whether LEMTRADA passes into breast milk, but there is a possibility that it may. It is recommended to interrupt breastfeeding during each treatment cycle with LEMTRADA and for 4 months after each treatment cycle. However, breast milk may be beneficial (can protect the baby from infections), so consult your doctor if you plan to breastfeed your baby, and they will advise you on the best course of action for you and your baby.
Fertility
LEMTRADA could remain in your body during the treatment cycle and up to 4 months after. It is unknown whether LEMTRADA has effects on fertility during this period. Consult your doctor if you are planning to have children. There is no evidence that LEMTRADA has an impact on male fertility.
Driving and using machines
Many patients experience side effects at the time of infusion with LEMTRADA or within 24 hours, and some of these effects, such as dizziness, may make it unsafe to drive or use machines. If this happens, stop these activities until you feel better.
LEMTRADAcontains potassium and sodium
This medicine contains potassium, less than 1 mmol of potassium(39 mg) per infusion; this is essentially "potassium-free".
This medicine contains less than 1 mmol of sodium(23 mg) per infusion; this is essentially "sodium-free".
3. How LEMTRADA will be administered
Your doctor will explain how LEMTRADA is administered. If in doubt, ask your doctor.
The initial treatmentyou will receive will consist of an infusion per day for 5 days (cycle 1) and one year later an infusion per day for 3 days (cycle 2).
Between the two cycles, you will not receive treatment with LEMTRADA. Two treatment cycles may reduce MS activity for up to 6 years.
Some patients, if they have symptoms or signs of MS disease after the two initial cycles, may receive one or two additional treatment cycles consisting of an infusion per day for 3 days. These additional treatment cycles can be administered 12 months or more after the previous treatment.
The maximum daily dose is one infusion.
LEMTRADA will be administered to you by infusion into a vein. Each infusion lasts about 4 hours. Monitoring for adverse effects and periodic testing should continue for 4 years after the last infusion.
To help you better understand the duration of the treatment effects and the duration of the necessary follow-up, see the following diagram.
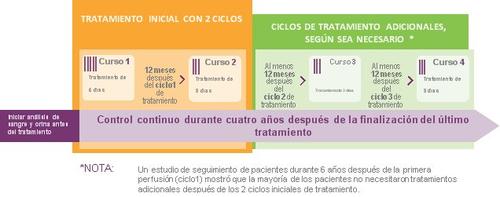
Follow-up after treatment with LEMTRADA
Once you have received LEMTRADA, you should undergo periodic tests so that any possible adverse effect can be diagnosed and treated quickly. These tests should continue until 4 years after the last infusion and are described in section 4, most important adverse effects.
If you receive more LEMTRADAthan you should
Patients who have been accidentally administered too much LEMTRADA in an infusion have experienced serious reactions, such as headache, rash, low blood pressure, or increased heart rate. Higher doses than recommended may cause more severe or longer-lasting infusion reactions (see section 4) or a greater effect on the immune system. Treatment consists of discontinuing the administration of LEMTRADA and treating the symptoms.
If you miss a dose of LEMTRADA
It is unlikely that you will forget your dose since it is administered by a healthcare professional. However, note that if a dose is missed, it should not be administered on the same day as a scheduled dose.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible adverse effects
Like all medicines, this medicine can cause adverse effects, although not all people experience them.
The most important adverse effectsare autoimmune diseasesdescribed in section 2, including:
- Acquired hemophilia A(uncommon - may affect up to 1 in 100 people): may show spontaneous hematomas, nosebleeds, pain or inflammation in joints, other types of bleeding or bleeding in case of cuts that may take longer than usual to stop.Immune thrombocytopenic purpura (ITP), (common - may affect up to 1 in 10 people): may present as small red, pink, or purple spots scattered on the skin; propensity to bruises; bleeding in case of cuts more difficult to stop; heavier, longer, or more frequent menstrual periods than usual; bleeding between menstrual periods; new or longer-than-usual bleeding from the gums or nose; or coughing up blood.
- Thrombotic thrombocytopenic purpura (TTP), (rare - may affect up to 1 in 1,000 people): may show hematomas on the skin or in the mouth, which may appear as small red dots, with or without extreme unexplained fatigue, fever, confusion, changes in speech, yellowing of the skin or eyes (jaundice), low urine output, or dark urine.
- Kidney disorders, (rare - may affect up to 1 in 1,000 people): may present as blood in the urine (urine may be red or tea-colored) or as swelling of the legs or feet. It can also cause lung damage and coughing up blood.
If you experience any of these signs or symptoms related to kidney or bleeding disorders, inform your doctor immediately. If you cannot contact your doctor, seek immediate medical attention.
- Thyroid disorders(very common - may affect more than 1 in 10 people): may present as excessive sweating; unexplained weight gain or loss; eye swelling; nervousness; rapid heartbeat; feeling cold; worsening fatigue; or new constipation.
- Red and white blood cell disorders(uncommon - may affect up to 1 in 100 people) diagnosed from blood tests.Sarcoidosis(uncommon - may affect 1 in 100 people): symptoms may consist of persistent dry cough, shortness of breath, chest pain, fever, inflammation of the lymph nodes, weight loss, skin rashes, and blurred vision.
- Autoimmune encephalitis(uncommon - may affect 1 in 100 people): may include symptoms such as behavioral and/or psychiatric changes, short-term memory loss, or seizures. The symptoms can resemble an MS relapse.
All these serious adverse effects can appear years after the administration of LEMTRADA. If you experience any of these signs or symptoms, inform your doctor immediately.You will also undergo blood and urine tests periodically to ensure that if you develop any of these diseases, they can be treated quickly.
Summary of the tests that will be performed to monitor the occurrence of autoimmune conditions:
Test | When? | Duration |
Blood test (to diagnose all serious adverse effects mentioned above) | Before starting treatment and every month after treatment | Up to 4 years after the last LEMTRADA infusion |
Urine test (additional test to diagnose kidney disorders) | Before treatment starts and every month when it ends | Up to 4 years after the last LEMTRADA infusion |
After this period, if you experience symptoms of ITP, acquired hemophilia A, TTP, thyroid or kidney disorders, your doctor will perform more tests. You should remain alert to the signs and symptoms of adverse effects beyond the four years as indicated in the Patient Guide, and you should also continue to carry the Patient Card with you.
Another important adverse effectis an increased risk of infections(see below for information on the frequency with which patients experience infections). In most cases, these infections are mild, but serious infectionscan occur.
If you experience any of these signs of infection,inform your doctor immediately
|
To help reduce the risk of some infections, your doctor may consider administering varicella and/or other vaccinations that they think you may need (see section 2: What you need to know before you start using LEMTRADA- Vaccinations). Your doctor may also prescribe a medication for mouth ulcers (see section 2: What you need to know before you start using LEMTRADA– Infections).
The most common adverse effectsare infusion reactions(see below for information on the frequency with which patients experience them), which can occur at the time of infusion or in the 24 hours following. In most cases, these reactions are mild but can be serious. Allergic reactions can also occur.
To try to reduce infusion reactions, your doctor will administer medications (corticosteroids) before each of the first 3 infusions of a LEMTRADA cycle. To limit these reactions, other treatments can also be administered before infusion or when symptoms are experienced. Additionally, you will be monitored during the infusion and up to 2 hours after it has finished. In case of serious reactions, the infusion can be slowed down or even stopped.
See the LEMTRADA patient guidefor more information on these events.
These are the adverse effectsyou may experience:
Very common(may affect more than 1 in 10 people):
- Infusion reactionsthat can occur at the time of infusion or in the 24 hours following: changes in heart rate, headache, rash, body rash, fever, urticaria, chills, itching, redness of the face and neck, feeling of fatigue, nausea
- Infections: respiratory tract infections such as colds and sinusitis, urinary tract infections, herpes infections
- Decrease in the number of white blood cells (lymphocytes, leukocytes, neutrophils)
- Thyroid disorders such as overactive or underactive thyroid gland
Common(may affect up to 1 in 10 people):
- Infusion reactionsthat can occur at the time of infusion or in the 24 hours following: indigestion, chest discomfort, pain, dizziness, altered taste, difficulty sleeping, difficulty breathing or shortness of breath, low blood pressure, pain at the infusion site
- Infections: cough, ear infection, flu-like illness, bronchitis, pneumonia, vaginal thrush or candidiasis, herpes, mouth ulcers, swollen or enlarged glands, flu, herpes zoster infection, tooth infection
- increase in the count of white blood cells such as neutrophils, eosinophils (different types of white blood cells), anemia, decrease in the percentage of red blood cells, easy or excessive bruising or bleeding, swollen lymph nodes
- exaggerated immune response
- back pain, neck, arm, or leg pain, muscle pain, muscle spasms, joint pain, mouth or throat pain
- inflammation of the mouth/gums/tongue
- general malaise, weakness, vomiting, diarrhea, abdominal pain, intestinal flu, hiccups
- abnormal liver test
- heartburn
- abnormalities that can be found during examination: blood or protein in the urine, decreased heart rate, irregular or abnormal heartbeat, high blood pressure, altered kidney function, white blood cells in the urine
- bruising
- MS relapse
- tremors, loss of sensation, burning or tingling sensation
- autoimmune thyroid gland disorder or goiter (inflammation of the thyroid gland in the neck)
- numbness of arms and/or legs
- vision problems, conjunctivitis, eye disease associated with thyroid disease
- feeling of dizziness or loss of balance, migraine
- feeling of anxiety, depression
- irregular, prolonged, or abnormally heavy menstrual periods
- acne, skin redness, excessive sweating, skin discoloration, skin lesion, dermatitis
- nosebleeds, bruising
- hair loss
- asthma
- muscle and bone pain, chest discomfort
Uncommon(may affect up to 1 in 100 people)
- Infections: intestinal flu, gum inflammation, fungal infection of the nails, tonsillitis, acute sinusitis, bacterial skin infection, cytomegalovirus infection
- neumonitis
- athlete's foot
- abnormal vaginal smear
- increased sensitivity, sensory disturbances such as numbness, tingling, and pain, tension headache
- double vision
- ear pain
- difficulty swallowing, throat irritation, productive cough
- weight loss, weight gain, decrease in red blood cells, increase in blood glucose, increase in red blood cell size
- constipation, acid reflux, dry mouth
- rectal bleeding
- gum bleeding
- decreased appetite
- blisters, night sweats, facial swelling, eczema
- stiffness, discomfort in arms and legs
- kidney stones, ketone bodies in the urine, kidney disease
- immune system weakness
- tuberculosis
- inflammation of the gallbladder with or without gallstones
- warts
- autoimmune disorder characterized by bleeding (acquired hemophilia A)
- sarcoidosis
- autoimmune brain disorder (autoimmune encephalitis)
- skin patches with loss of color (vitiligo)
- autoimmune hair loss (alopecia areata)
Rare(may affect up to 1 in 1,000 people)
- excessive activation of white blood cells associated with inflammation (hemophagocytic lymphohistiocytosis)
- autoimmune blood coagulation disorder (thrombotic thrombocytopenic purpura, TTP)
Frequency not known(cannot be estimated from the available data)
- listeriosis/listeria meningitis
- bleeding in the lungs
- heart attack
- stroke
- tears in the vertebral or carotid arteries (blood vessels that supply blood to the brain)
- infection with the Epstein-Barr virus
- inflammatory disease that affects multiple organs, adult-onset Still's disease (AOSD)
Show the Patient Card and this leaflet to any doctor involved in your treatment and not just the neurologist.
You will also find this information in the Patient Card and the patient guide provided by your doctor.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of LEMTRADA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box and the vial label after EXP. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Keep in the original packaging to protect it from light.
It is recommended to use the product immediately after dilution due to the possible risk of microbial contamination. If not used immediately, the conditions and storage times used before use are the responsibility of the user and should not exceed 8 hours at a temperature of 2°C to 8°C, protected from light.
6. Container Contents and Additional Information
LEMTRADA Composition
The active substanceis alemtuzumab.
Each vial contains 12 mg of alemtuzumab in 1.2 ml.
Other components are:
- disodium phosphate dihydrate (E339)
- disodium edetate dihydrate
- potassium chloride (E508)
- potassium dihydrogen phosphate (E340)
- polysorbate 80 (E433)
- sodium chloride
- water for injectable preparations
Appearance ofLEMTRADAand Container Contents
LEMTRADA is a concentrate for solution for infusion (sterile concentrate) that is clear and colorless or slightly yellow, supplied in a glass vial with a stopper.
There is 1 vial in each carton.
Marketing Authorization Holder
Sanofi Belgium
Leonardo Da Vincilaan 19
B-1831 Diegem
Belgium
Manufacturer
Genzyme Ireland Limited
IDA Industrial Park
Old Kilmeaden Road
Waterford
Ireland
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
België/Belgique/Belgien/Luxemburg/Luxembourg Sanofi Belgium Tél/Tel: + 32 2 710 54 00 | Lietuva Swixx Biopharma UAB Tel. +370 5 236 91 40 |
| Magyarország SANOFI-AVENTIS Zrt Tel: +36 1 505 0050 |
Ceská republika sanofi-aventis, s.r.o. Tel: +420 233086 111 | Malta Sanofi S..l. Tel: +39 02 39394275 |
Danmark Sanofi A/S Tlf: +45 45 16 70 00 | Nederland Sanofi B.V. Tel: +31 20 245 4000 |
Deutschland Sanofi Belgium Tel: +49 (0) 6102 3674 451 | Norge sanofi-aventis Norge AS Tlf: + 47 67 10 71 00 |
Eesti Swixx Biopharma OÜ Tel. +372 640 10 30 | Österreich sanofi-aventis GmbH Tel: + 43 1 80 185 - 0 |
Ελλάδα Sanofi-Aventis Μονοπρ?σωπη AEBE Τηλ: +30 210 900 16 00 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Portugal Sanofi – Produtos Farmacêuticos, Lda. Tel: +351 21 35 89 400 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Appel depuis l’étranger: +33 1 57 63 23 23 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma Tel.: +421 2 208 33 600 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44353 (0) 403 56 00 | Suomi/Finland Sanofi Oy Puh/Tel: + 358 201 200 300 |
Italia Sanofi S.r.l.. Tel: 800536389 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Κύπρος C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | United Kingdom (Northern Ireland) sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +44 (0) 800 035 2525 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50CP |
Date of Last Revision of this Leaflet:
Other Sources of Information
To help educate patients about the possible adverse effects and instructions on what to do if certain adverse effects occur, the following risk minimization materials are available:
1 Patient Card: For the patient to present to other healthcare professionals to alert them to the patient's use of LEMTRADA.
2 Patient Guide: For more information on autoimmune reactions, infections, and other information.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
--------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
Risk Minimization Information – Autoimmune Conditions
- It is extremely important that the patient understands the commitment to undergoing periodic tests (for 4 years after the last infusion) even if they have no symptoms and the disease is well-controlled.
- Together with the patient, you need to plan and manage the periodic checks.
- Non-compliant patients may need additional counseling to highlight the risks of missing scheduled periodic tests.
- You should monitor the test results and remain alert to any symptoms of an adverse reaction.
- Review the LEMTRADA Summary of Product Characteristics and Patient Guide with your patient. Remind the patient to be alert to any symptoms related to autoimmune conditions and to seek medical help if in doubt.
There are also educational materials available for healthcare professionals:
- LEMTRADA Healthcare Professional Guide
- LEMTRADA Training Module
- LEMTRADA Prescriber Checklist
Read the Summary of Product Characteristics (available on the EMA website mentioned above) for more information.
Information for Preparing LEMTRADA Administration and Monitoring the Patient
- Patients should be pre-medicated with corticosteroids immediately before LEMTRADA infusion during the first 3 days of any treatment cycle. Pre-treatment with antihistamines and/or antipyretics before LEMTRADA administration may also be considered.
- During treatment and up to 1 month after, an oral antiherpetic should be administered to all patients. In clinical trials, 200 mg of aciclovir was administered twice daily or equivalent to patients.
- Baseline comprehensive tests and screenings are described in Section 4 of the Summary of Product Characteristics.
- The vial contents should be visually inspected before each administration for particulate matter or discoloration. If the concentrate contains particulate matter or is discolored, it should not be used. DO NOT SHAKE THE VIALS BEFORE USE.
- Follow aseptic techniques to withdraw 1.2 ml of LEMTRADA from the vial and inject into 100 ml of a 9 mg/ml (0.9%) sodium chloride solution for infusion or a glucose solution for infusion (5%). The bag should be gently inverted to mix the solution. Care should be taken to ensure the sterility of the prepared solution.
- Administer the LEMTRADA infusion solution intravenously over a period of approximately 4 hours.
- No other medicinal products should be added to the LEMTRADA infusion solution or infused simultaneously through the same intravenous line.
- The product should be used immediately after dilution due to the potential risk of microbial contamination. If not used immediately, the storage conditions and times before use are the responsibility of the user and should not exceed 8 hours at a temperature of 2°C to 8°C, protected from light.
- Appropriate handling and disposal procedures should be followed. Disposal of any unused material or waste should be in accordance with local requirements.
After each infusion, the patient should be monitored for 2 hours to detect any infusion-related reactions. Symptomatic treatment may be initiated if necessary - see Summary of Product Characteristics. Continue to perform analytical tests on the patient every month to detect autoimmune diseases, up to 4 years after the last infusion. Consult the LEMTRADA Healthcare Professional Guide for more information, or read the Summary of Product Characteristics available on the EMA website mentioned above.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEMTRADA 12 mg concentrate for infusion solutionDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE, 200 mgActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for LEMTRADA 12 mg concentrate for infusion solution
Discuss questions about LEMTRADA 12 mg concentrate for infusion solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







