
LAVENTAIR ELLIPTA 55 micrograms/22 micrograms INHALATION POWDER (single dose)

How to use LAVENTAIR ELLIPTA 55 micrograms/22 micrograms INHALATION POWDER (single dose)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
LAVENTAIR ELLIPTA55micrograms/22microgramspowder for inhalation (single dose)
umeclidinium/vilanterol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What LAVENTAIR ELLIPTA is and what it is used for
- What you need to know before you use LAVENTAIR ELLIPTA
- How to use LAVENTAIR ELLIPTA
- Possible side effects
- Storing LAVENTAIR ELLIPTA
- Contents of the pack and other information
Step-by-step instructions for use
1. What LAVENTAIR ELLIPTA is and what it is used for
What LAVENTAIR ELLIPTA is
LAVENTAIR ELLIPTA contains two active substances, umeclidinium bromide and vilanterol. These belong to a group of medicines called bronchodilators.
What LAVENTAIR ELLIPTA is used for
LAVENTAIR ELLIPTA is used to treat chronic obstructive pulmonary disease (COPD) in adults. COPD is a long-term disease that gets slowly worse and causes difficulty breathing.
In COPD, the muscles around the airways contract. This medicine prevents the contraction of these muscles in the lungs, making it easier to breathe in and out.
When used regularly, it helps control breathing difficulties and reduces the effects of COPD on daily life.
LAVENTAIR ELLIPTA should not be used to relieve a sudden attack of shortness of breath or wheezing (whistling sound when breathing).If you have this type of attack, you should use a fast-acting "rescue" inhaler (such as salbutamol). If you do not have a fast-acting inhaler, contact your doctor.
2. What you need to know before you use LAVENTAIR ELLIPTA
Do not use LAVENTAIR ELLIPTA:
- if you are allergic to umeclidinium, vilanterol, or any of the other ingredients of this medicine (listed in section 6).
If you think any of the above applies to you, do not usethis medicine until you have consulted your doctor.
Warnings and precautions
Talk to your doctor before you start using this medicine:
- if you have asthma(Do not use LAVENTAIR ELLIPTA for the treatment of asthma)
- if you have heart problemsor high blood pressure
- if you have a type of eye problem called narrow-angle glaucoma
- if you have enlarged prostate, difficulty urinating, or a blockage in the bladder
- if you have epilepsy
- if you have thyroid problems
- if you have low potassium levels in the blood
- if you have diabetes
- if you have severe liver problems.
Talk to your doctorif you think any of the above applies to you.
Urgent breathing difficulties
If you get chest tightness, coughing, wheezing, or difficulty breathing immediately after using your LAVENTAIR ELLIPTA inhaler:
stop using this medicine and seek medical help immediately, as you may have a serious condition called paradoxical bronchospasm.
Eye problems during treatment with LAVENTAIR ELLIPTA
If you get eye pain or discomfort, blurred vision for a while, halos around lights, or colored images associated with redness of the eyes during treatment with LAVENTAIR ELLIPTA:
stop using this medicine and seek medical help immediately, as these signs may be due to a sudden attack of narrow-angle glaucoma.
Children and adolescents
Do not give this medicine to children or adolescents under 18 years.
Other medicines and LAVENTAIR ELLIPTA
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. If you are not sure what your medicine contains, ask your doctor or pharmacist.
Some medicines may affect how this medicine works or make it more likely that you will get side effects. These include:
- medicines called beta-blockers (such as propranolol), used to treat high blood pressureor other heart conditions
- ketoconazole or itraconazole, to treat fungal infections
- clarithromycin or telithromycin, to treat bacterial infections
- ritonavir, to treat HIV
- medicines that lower potassium levels in the blood, such as some diuretics or some medicines to treat asthma (such as methylxanthines or steroids)
- other long-acting medicines to treat breathing problems similar to this medicine, such as tiotropium, indacaterol. Do not use LAVENTAIR ELLIPTA if you are already using these medicines.
Talk to your doctor or pharmacistif you are taking any of these medicines. Your doctor may monitor you closely if you are taking any of these medicines, as they may increase the side effects of LAVENTAIR ELLIPTA.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctorfor advice before using this medicine. If you are pregnant, do not use this medicine unless your doctor tells you to.
It is not known if the ingredients of LAVENTAIR ELLIPTA are excreted in breast milk. If you are breastfeeding, talk to your doctorbefore using LAVENTAIR ELLIPTA. If you are breastfeeding, do not use this medicine unless your doctor tells you to.
Driving and using machines
LAVENTAIR ELLIPTA is unlikely to affect your ability to drive or use machines.
LAVENTAIR ELLIPTA contains lactose
If your doctor has told you that you have an intolerance to some sugars, talk to your doctor before using this medicine.
3. How to use LAVENTAIR ELLIPTA
Use this medicine exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist.
The recommended doseis one inhalation every day, at the same time each day. You only need one inhalation a day, as the effect of this medicine lasts for 24 hours.
Do not use more doses than your doctor has recommended.
Use LAVENTAIR ELLIPTA regularly
It is very important that you use LAVENTAIR ELLIPTA every day, as your doctor has told you. This will help you not have symptoms throughout the day and night.
Do notuse LAVENTAIR ELLIPTA to relieve a sudden attack of shortness of breath or wheezing. If you have this type of attack, you should use a fast-acting "rescue" inhaler (such as salbutamol).
How to use the inhaler
To get the full instructions, read the "Step-by-step instructions for use" at the end of this leaflet.
LAVENTAIR ELLIPTA is for inhalation use only. To use LAVENTAIR ELLIPTA, breathe it into your lungs through your mouth using the ELLIPTA inhaler.
If your symptoms do not improve
If your COPD symptoms (shortness of breath, wheezing, coughing) do not improve or get worse, or if you are using your fast-acting "rescue" inhaler more often than usual:
contact your doctor as soon as possible.
If you use more LAVENTAIR ELLIPTA than you should
If you accidentally use too much medicine, contact your doctor or pharmacist immediately, as you may need medical attention. If possible, show them the inhaler, packaging, or this leaflet. You may notice that your heart beats faster than normal, have changes in vision, dry mouth, or headache.
If you forget to use LAVENTAIR ELLIPTA
Do not inhale a double dose to make up for forgotten doses.Inhale the next dose at your usual time. If you have wheezing or shortness of breath, use your fast-acting "rescue" inhaler (such as salbutamol) and seek medical advice.
If you stop using LAVENTAIR ELLIPTA
Use this medicine for as long as your doctor recommends. It will only be effective as long as you use it. Do not stop using it until your doctor tells you to, even if you feel better, as your symptoms may get worse.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions
If you get any of the following symptoms after using LAVENTAIR ELLIPTA, stop using this medicine and tell your doctor immediately.
Uncommon side effects (may affect up to 1 in 100 people):
- skin rash (hives) or redness.
Rare side effects (may affect up to 1 in 1,000 people):
- swelling, sometimes of the face or mouth (angioedema)
- increased wheezing, coughing, or difficulty breathing
- sudden feeling of weakness or dizziness (which may cause collapse or loss of consciousness).
Urgent breathing difficultiesUrgent breathing difficulties after using LAVENTAIR ELLIPTA are rare. If you get chest tightness, coughing, wheezing, or difficulty breathing immediately after using this medicine:
stop using this medicine and seek medical help immediately, as you may have a serious condition called paradoxical bronchospasm.
Other side effects
Common (may affect up to 1 in 10 people)
- painful or frequent urination (may be a sign of urinary tract infection)
- combination of sore throat and runny nose
- sore throat
- feeling of pressure or pain in the cheeks and forehead (may be a sign of sinusitis)
- headache
- coughing
- pain or irritation in the back of the mouth and throat
- constipation
- dry mouth
- upper respiratory tract infection.
Uncommon (may affect up to 1 in 100 people)
- irregular heartbeat
- faster heartbeat
- feeling your heartbeat (palpitations)
- muscle spasms
- shaking
- change in taste
- hoarseness.
Rare (may affect up to 1 in 1,000 people)
- blurred vision
- increased eye pressure
- decreased vision or eye pain (possible signs of glaucoma)
- difficulty or pain when urinating, these may be signs of bladder obstruction or urinary retention.
Not known (frequency cannot be estimated from the available data)
- dizziness.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing LAVENTAIR ELLIPTA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the pack, tray, and inhaler, after EXP. The expiry date is the last day of the month shown.
Keep the inhaler in the sealed tray to protect it from moisture and only remove it immediately before first use. Once the tray is opened, the inhaler can be used for a period of 6 weeks, starting from the date of opening the tray. Write the date that the inhaler should be discarded on the space provided on the inhaler label. The date should be written as soon as the inhaler is removed from the tray.
Do not store above 30°C.
If stored in a refrigerator, allow the inhaler to return to room temperature for at least one hour before use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container contents and additional information
Composition ofLAVENTAIRELLIPTA
The active ingredients are umeclidinium bromide and vilanterol.
Each inhalation provides a delivered dose (dose that comes out of the mouthpiece) of 55 micrograms of umeclidinium (equivalent to 65 micrograms of umeclidinium bromide) and 22 micrograms of vilanterol (as trifenatate).
The other ingredients are lactose monohydrate (see section “LAVENTAIR ELLIPTA contains lactose” in section 2) and magnesium stearate.
Appearance of the product and container contents
LAVENTAIR ELLIPTA is a powder for inhalation (single-dose).
The Ellipta inhaler device consists of a light gray plastic body, a red mouthpiece cover, and a dose counter. It is packaged in a laminated aluminum tray. The tray contains a desiccant sachet to reduce the moisture in the container.
The active ingredients are presented as a white powder in separate blisters within the inhaler. LAVENTAIR ELLIPTA is available in packs of 1 inhaler containing 7 or 30 doses and in multipacks containing 90 doses (3 inhalers of 30 doses). Not all pack sizes may be marketed.
Marketing authorization holder:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
Ireland
D24 YK11
Manufacturer:
Glaxo Wellcome Production
Zone Industrielle No.2,
23 Rue Lavoisier,
27000 Evreux,
France
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tel: + 32 (0) 10 85 52 00 | Lietuva UAB “BERLIN-CHEMIE MENARINI BALTIC” Tel: +370 52 691 947 |
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgique/Belgien Tél/Tel: + 32 (0) 10 85 52 00 |
Ceská republika GlaxoSmithKline, s.r.o. Tel: + 420 222 001 111 | Magyarország Berlin-Chemie/A. Menarini Kft. Tel.: +36 23501301 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Limited Tel: +356 80065004 |
Deutschland BERLIN-CHEMIE AG Tel.: + 49 (0) 30 67070 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti OÜ Berlin-Chemie Menarini Eesti Tel: +372 667 5001 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλáδα Guidotti Hellas A.E. Τηλ: + 30 210 8316111-13 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España FAES FARMA, S.A. Tel: + 34 900 460 153 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France MENARINI France Tél: + 33 (0)1 45 60 77 20 | Portugal BIAL, Portela & Ca. SA. Tel: + 351 22 986 61 00 |
Hrvatska Berlin-Chemie Menarini Hrvatska d.o.o. Tel: +385 1 4821 361 | România GlaxoSmithKline Trading Services Limited Tel: +40 800672524 |
Ireland GlaxoSmithKline Trading Services Limited Tel: + 353 (0)1 4955000 | Slovenija Berlin-Chemie / A. Menarini Distribution Ljubljana d.o.o. Tel: +386 (0)1 300 2160 |
Ísland Vistor hf. Sími: + 354 535 7000 | Slovenská republika Berlin-Chemie / A. Menarini Distribution Slovakia s.r.o. Tel: +421 2 544 30 730 |
Italia A.Menarini Industrie Farmaceutiche Riunite s.r.l. Tel: + 39 (0)55 56801 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 |
Κúπρος GlaxoSmithKline Trading Services Limited Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija SIA Berlin-Chemie/Menarini Baltic Tel: +371 67103210 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Step-by-step instructions for use
What is the ELLIPTA inhaler?
The first time you use LAVENTAIR ELLIPTA, you do not need to check that the inhaler is working correctly, as it contains pre-measured doses and is ready to use directly.
Your LAVENTAIR ELLIPTA inhaler contains:
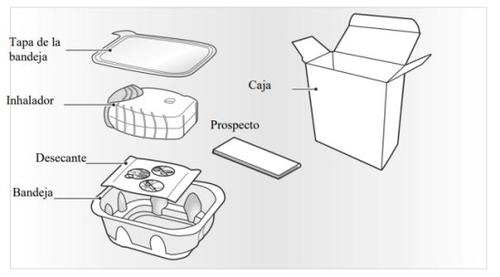
The inhaler is packaged in a tray. Do not open the tray until you are ready to start using your new inhaler. When you are ready to use the inhaler, remove the lid to open the tray. The tray contains a desiccantsachet, to reduce the moisture in the container.
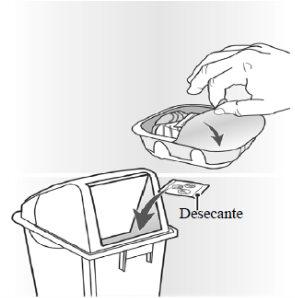
When you remove the inhaler from its tray, it will be in the “closed” position. Do not open the inhaler until you are ready to inhale a dose of the medicine. When the tray is opened, you should write the “Discard by” date on the space provided on the inhaler label. The “Discard by” date is 6 weeks from the date of opening the tray.
After this date, the inhaler should not be used anymore. The tray can be discarded after the first opening.
If stored in the refrigerator, let the inhaler reach room temperature for at least one hour before use.
The step-by-step instructions for using the inhaler provided below can be used for both the 30-dose inhaler (30 days of treatment) and the 7-dose inhaler (7 days of treatment).
- Read this information before starting
If the inhaler cap is opened and closed without inhaling the medicine, the dose will be lost. The lost dose will be retained safely inside the inhaler, but it will not be available for inhalation.
It is not possible to accidentally administer an extra dose or a double dose through an inhalation.
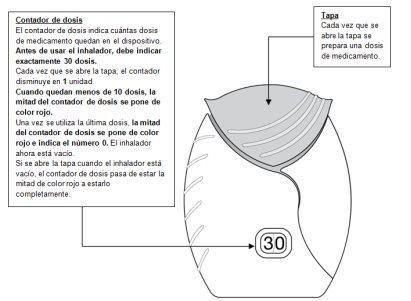
- Prepare a dose
Wait to open the inhaler cap until you are ready to inhale a dose.
Do not shake the inhaler.
- Slide the cap down until you hear a ‘click’.
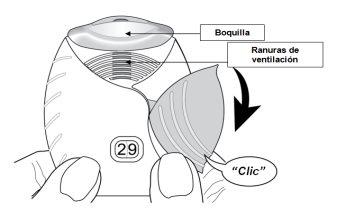
Now, the medicine is ready to be inhaled.
As confirmation, the dose counter decreases by 1unit.
- If the dose counter does not decrease when you hear the ‘click’, the inhaler will not release the dose of the medicine.
Take it to the pharmacist and ask for help.
- Inhale your medicine
- While keeping the inhaler away from your mouth, breathe out as much as you can.
Do notbreathe out into the inhaler.
- Place the mouthpiece between your lips, and close them firmly around the mouthpiece.
Do notblock the ventilation slots with your fingers.

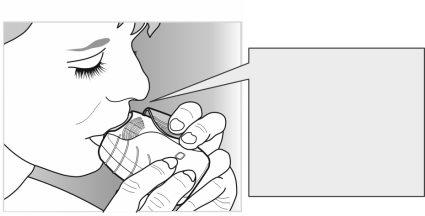
- Take a long, steady, and deep breath in. Hold your breath for as long as you can (at least 3-4 seconds).
- Remove the inhaler from your mouth.
- Breathe out slowly and gently.
You may not be able to taste or feel the medicine, even when using the inhaler correctly.
Beforeclosing the cap, the inhaler mouthpiece can be cleaned using a dry tissue.
- Close the inhaler
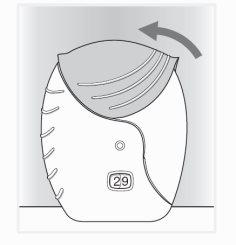
Slide the cap up, until it clicks, to cover the mouthpiece.
- Country of registration
- Average pharmacy price70.25 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LAVENTAIR ELLIPTA 55 micrograms/22 micrograms INHALATION POWDER (single dose)Dosage form: PULMONARY INHALATION, 55 MICROGRAMS/22 MICROGRAMSActive substance: vilanterol and umeclidinium bromideManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: PULMONARY INHALATION, 340/12 microgramsActive substance: formoterol and aclidinium bromideManufacturer: Covis Pharma Europe B.V.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Genetic S.P.A.Prescription required
Online doctors for LAVENTAIR ELLIPTA 55 micrograms/22 micrograms INHALATION POWDER (single dose)
Discuss questions about LAVENTAIR ELLIPTA 55 micrograms/22 micrograms INHALATION POWDER (single dose), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















