
KYBERNIN P 1000 IU POWDER AND SOLVENT FOR SOLUTION FOR INJECTION OR INFUSION

How to use KYBERNIN P 1000 IU POWDER AND SOLVENT FOR SOLUTION FOR INJECTION OR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Kybernin P 1000 UI powder and solvent for solution for injection and infusion.
Human Antithrombin III
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.Keep this leaflet, you may need to read it again.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Kybernin P and what is it used for
- What you need to know before you use Kybernin P
- How to use Kybernin P
- Possible side effects
- Storage of Kybernin P
- Contents of the pack and other information
1. What is Kybernin P and what is it used for
Kybernin P is a powder and solvent for solution for injection and infusion.
This medicine belongs to a group of medicines called antithrombotic agents.
Kybernin P is used if you have a congenital deficiency of antithrombin, to prevent the formation and development of blood clots in the blood vessels of your legs (deep vein thrombosis) or in other blood vessels of your body (thromboembolism) during surgery or in the peri-partum period and in association with heparin if indicated.
Kybernin P is also used if you have an acquired deficiency of antithrombin.
2. What you need to know before you use Kybernin P
Do not use Kybernin P:
If you are allergic to the active substance or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
As with any protein product for intravenous administration, hypersensitivity reactions of an allergic type may occur. Close monitoring and careful observation of patients are required to detect any symptoms during the infusion period. Patients should be informed about the initial signs of hypersensitivity reactions, which include skin rashes that can lead to generalized urticaria, chest tightness, difficulty breathing, hypotension, and anaphylaxis. If these symptoms occur after administration, contact your doctor.
In case of shock, the current recommendations for the treatment of shock should be followed.
Viral safety
When human blood or plasma-derived medicines are administered, certain measures must be taken to prevent infections from being passed on to patients. Such measures include:
- Careful selection of donors to exclude those who are at risk of being carriers of infectious diseases,
- Testing for specific infection markers in individual donations and plasma pools,
- Inclusion of stages in the manufacturing process to eliminate/inactivate viruses.
Despite this, when human blood or plasma-derived medicines are administered, the possibility of transmitting infectious agents cannot be completely ruled out. This also applies to emerging or unknown viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and for non-enveloped viruses such as hepatitis A (HAV) and parvovirus B19.
Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly receive products with antithrombin derived from human plasma.
It is highly recommended that each time Kybernin P is administered to a patient, a record is kept of the name of the medicine and batch number administered in order to maintain a relationship between the patient and the product batch.
Clinical and biological monitoring in case of concomitant administration of antithrombin and heparin:
- In order to adjust the heparin dose and avoid excessive hypocoagulability, regular controls of the extent of anticoagulation (APTT, and when appropriate anti-FXa activity) should be performed at short intervals, especially in the first minutes/hours after the start of antithrombin administration.
- Daily determination of antithrombin levels, in order to adjust the individual dose, due to the risk of decreased antithrombin levels as a consequence of prolonged treatment with unfractionated heparin.
Use of Kybernin P with other medicines
Heparin: Replenishment of antithrombin during administration of heparin in therapeutic doses increases the risk of bleeding. The effect of antithrombin is greatly potentiated by heparin. The half-life of antithrombin may be significantly reduced by concomitant treatment with heparin, due to accelerated mobilization of antithrombin. Consequently, the simultaneous administration of heparin and antithrombin to a patient at high risk of bleeding should be clinically and biologically monitored.
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
Experience with the safety of human antithrombin products for use in human pregnancy is limited.
The safety of using Kybernin P in human pregnancy has not been established in controlled clinical trials. Animal studies are insufficient to assess safety with respect to reproduction, embryo or fetal development, the course of pregnancy, and peri- and postnatal development.
There are no negative experiences related to treatment during pregnancy and breastfeeding.
Therefore, Kybernin P should be administered to pregnant or breastfeeding women with antithrombin deficiency only if it is clearly indicated, taking into account that pregnancy confers an increased risk of thromboembolic episodes in these patients.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. Your doctor will weigh the possible risk to the fetus and inform you if treatment with this medicine is appropriate. Your doctor will only recommend this treatment if it is clearly indicated.
Driving and using machines
There is no indication that Kybernin P may affect the ability to drive or use machines.
Kybernin P contains sodium
Patients on low-sodium diets should note that Kybernin P 1000 UI contains up to 89.52 mg (3.894 mmol) of sodium per 1000 UI.
3. How to use Kybernin P
Kybernin P is a hospital medicine, so it will be administered to you in a hospital by the corresponding healthcare staff.
Kybernin P is administered by preparing a solution beforehand, which is injected or infused intravenously slowly (maximum 4 ml/min).
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Your doctor will indicate how often and at what intervals Kybernin P should be administered.
Your doctor will indicate the duration of your treatment with Kybernin P.
If you use more Kybernin P than you should:
No symptoms of overdose with antithrombin have been reported.
In case of overdose or accidental administration, consult the Toxicology Information Service. Telephone 91 562 04 20.
If you forget to use Kybernin P:
- Consult your doctor or pharmacist immediately.
- Do not administer a double dose to make up for forgotten doses.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following adverse reactions are based on post-marketing experience. In cases where data are available, the following standard frequency categories have been used:
Very common >1/10
Common >1/100 to <1>
Uncommon ≥ 1/1,000 to <1>
Rare ≥ 1/10,000 to <1>
Very rare <1>
Classification by Organ and System | Preferred Term | Frequency |
Disorders of the immune system | Hypersensitivity/anaphylactic reactions, including severe anaphylaxis and shock. | Rare |
General disorders and administration site conditions | Pyrexia | Rare |
For information on viral safety, see “Warnings and precautions” in section 2 of this leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Kybernin P
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Do not freeze.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month shown.
Do not use solutions that are turbid or contain deposits (sediment/particles).
After reconstitution, the physical-chemical stability has been demonstrated for a period of 8 hours at room temperature (max. 25°C). From a microbiological point of view and given that Kybernin P does not contain preservatives, the reconstituted solution should be used immediately. If this is not possible, do not store for more than 8 hours at room temperature (maximum 25°C).
Disposal of unused medicine or waste material should be done in accordance with local regulations.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Kybernin P 1,000 UI
- The active substance is antithrombin III. Each vial of lyophilized powder contains 1,000 UI of antithrombin III. The reconstituted solution contains approximately 50 UI of antithrombin III/ml of human plasma-derived antithrombin when reconstituted with 20 ml of water for injections.
The potency (UI) is determined using the chromogenic substrate method according to the European Pharmacopoeia. The specific activity of Kybernin P is approximately 5.3 UI/mg of protein.
- The other ingredients are: glycine, sodium chloride, sodium citrate, hydrochloric acid or sodium hydroxide (for pH adjustment), and water for injections.
See section 2 for important information about some of the excipients.
Appearance of the product and pack contents
Powder and solvent for solution for injection and infusion.
The commercial pack contains a glass vial of type II (according to Eur. Ph.), colorless and sealed with a rubber stopper, plastic disc, and aluminum cap containing the lyophilized powder, a vial with 20 ml of water for injections (solvent for preparation of the solution), and a transfer device.
Presentation:
Individual pack of Kybernin P 1,000 UI:
1 vial of lyophilized powder
1 vial with 20 ml of water for injections
1 transfer device
Clinical pack of Kybernin P 1,000 UI:
10 vials of lyophilized powder
10 vials with 20 ml of water for injections
10 transfer devices
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
CSL Behring, S.A.
c/ Tarragona 157, planta 18
08014 Barcelona - Spain
Manufacturer
CSL Behring GmbH
Emil-von-Behring-Str. 76
35041 Marburg - Germany
Date of last revision of this leaflet: November 2020
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
This information is intended only for healthcare professionals:
Dosage
In congenital deficiency, the dose should be individualized for each patient, taking into account the family history regarding thromboembolic episodes, the patient's clinical risk factors, and laboratory tests.
The dosing and duration of replacement therapy in acquired deficiency depend on the plasma antithrombin level, the presence of signs of increased mobilization, the underlying disorder, and the severity of the patient's clinical condition. The dose and frequency of administration should always be based on clinical efficacy and laboratory tests in each individual case.
The number of antithrombin units administered is expressed in International Units (UI), in relation to the World Health Organization (WHO) standard for antithrombin. Plasma antithrombin activity is expressed as a percentage (in relation to normal human plasma) or in International Units (in relation to an international standard for antithrombin in plasma).
One International Unit (UI) of antithrombin activity is equivalent to the amount of antithrombin in 1 ml of normal human plasma. The calculation of the required dose of antithrombin is based on the empirical finding that 1 International Unit (UI) of antithrombin per kg of body weight increases plasma antithrombin activity by approximately 1.5%.
The initial dose is determined using the following formula:
Units required = body weight [kg] x (100 – current antithrombin activity [%]) x 2/3
The antithrombin activity to be achieved initially depends on the clinical condition. When substitution with antithrombin is indicated, the dose should be sufficient to achieve the desired antithrombin activity and maintain an effective level. The dose should be determined and monitored according to laboratory tests of antithrombin activity, which should be performed at least twice a day until the patient is stabilized, and then once a day, preferably immediately before the next infusion. The dose adjustment should take into account both the signs of increased antithrombin production according to laboratory tests and the clinical evolution. Antithrombin activity should be maintained above 80% during treatment, unless the clinical condition indicates a different level of efficacy.
The usual initial dose in congenital deficiency is 30-50 UI/kg.
Therefore, the dose and frequency of administration, as well as the duration of treatment, should be adjusted to the biological data and clinical situation.
Pediatric population:
Kybernin P is not recommended for use in children under 6 years of age due to the scarcity of data.
Based on clinical experience, the use of antithrombin cannot be recommended for the treatment of Respiratory Distress Syndrome (RDS) in premature infants.
Instructions for the correct administration of the preparation
General instructions
The lyophilized powder must be completely reconstituted, under aseptic conditions, with the accompanying solvent. A clear or slightly opalescent solution is obtained.
The suitable diluent is a 5% human albumin solution. For dilutions of up to 1:5, the following may also be used: Ringer's lactate solution, physiological saline solution, 5% glucose solution, or polygeline.
The use of hydroxyethyl starches is not recommended as a solvent (for infusion), as a loss of antithrombin activity has been observed.
This medicine should not be mixed with other medicines in the syringe/infusion equipment. Dopamine, dobutamine, and furosemide should not be administered through the same venous access.
The product should be administered intravenously. Maximum infusion rate: 4 ml/min.
Reconstitution
To correctly handle the double-pointed Transofix, follow the steps below:
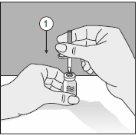
- After removing one of the two protective caps, insert the exposed point perpendicularly into the rubber stopper of the solvent vial.
- Remove the protective cap from the second point.
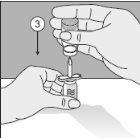
- Puncture the head of the product vial with this point.
- Turn the entire unit 180°.
- Place it so that the base of the product vial is supported on the surface of the table. The solvent now flows into the product vial.
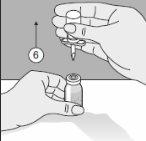
- The double-pointed Transofix along with the solvent vial is removed from the product vial, and then Kybernin P is dissolved. The reconstituted Kybernin P can be drawn into a syringe and administered.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KYBERNIN P 1000 IU POWDER AND SOLVENT FOR SOLUTION FOR INJECTION OR INFUSIONDosage form: INJECTABLE PERFUSION, 1,000 IU Human Antithrombin IIIActive substance: antithrombin IIIManufacturer: Octapharma S.A.Prescription requiredDosage form: INJECTABLE PERFUSION, 500 IU human antithrombin IIIActive substance: antithrombin IIIManufacturer: Octapharma S.A.Prescription requiredDosage form: INJECTABLE, 500 IU antithrombinActive substance: antithrombin IIIManufacturer: Csl Behring S.A.Prescription required
Online doctors for KYBERNIN P 1000 IU POWDER AND SOLVENT FOR SOLUTION FOR INJECTION OR INFUSION
Discuss questions about KYBERNIN P 1000 IU POWDER AND SOLVENT FOR SOLUTION FOR INJECTION OR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















