KESIMPTA 20 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN

How to use KESIMPTA 20 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Kesimpta 20 mg solution for injection in pre-filled pen
ofatumumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Kesimpta and what is it used for
- What you need to know before you use Kesimpta
- How to use Kesimpta
- Possible side effects
- Storage of Kesimpta
- Contents of the pack and other information
1. What is Kesimpta and what is it used for
What is Kesimpta
Kesimpta contains the active substance ofatumumab. Ofatumumab belongs to a group of medicines known as monoclonal antibodies.
What Kesimpta is used for
Kesimpta is used to treat adults with relapsing forms of multiple sclerosis (RMS).
How Kesimpta works
Kesimpta works by binding to a target known as CD20, which is on the surface of B lymphocytes. B lymphocytes are a type of white blood cell that is part of the immune system (the body's defenses). In multiple sclerosis, the immune system attacks the protective layer around nerve cells. B lymphocytes are involved in this process. Kesimpta targets B lymphocytes and eliminates them. This reduces the likelihood of a relapse, alleviates symptoms, and slows down the progression of the disease.
2. What you need to know before you use Kesimpta
Do not use Kesimpta
- if you are allergic to ofatumumab or any of the other ingredients of this medicine (listed in section 6).
- if you have been diagnosed with severe immune system problems.
- if you have a severe infection.
- if you have cancer.
Warnings and precautions
Talk to your doctor before you start using Kesimpta
- Kesimpta may cause the hepatitis B virus to become active again. Your doctor will perform a blood test to check if you are at risk of hepatitis B infection. If the result shows that you have had hepatitis B or that you are a carrier of the hepatitis B virus, your doctor will ask you to visit a specialist.
- Before starting treatment with Kesimpta, your doctor may check your immune system.
- If you have an infection, your doctor may decide that you cannot use Kesimpta or that you must delay treatment with Kesimpta until the infection is resolved.
- Your doctor will check if you need any vaccinations before starting treatment with Kesimpta. If you need a type of vaccine known as a live or attenuated vaccine, it should be given at least 4 weeks before starting treatment with Kesimpta. Other types of vaccines should be given at least 2 weeks before starting treatment with Kesimpta.
While using Kesimpta
Tell your doctor:
- if you experience a general reaction related to the injection or a local reaction at the injection site. These are the most common side effects of treatment with Kesimpta and are described in section 4. They usually occur within 24 hours after injecting Kesimpta, especially after the first injection. The first injection should be performed under the guidance of a healthcare professional.
- if you have an infection. You may be more likely to get infections or if you already have an infection, it could get worse. This is because the immune cells that Kesimpta acts on also help fight infections. Infections can be serious and sometimes life-threatening.
- if you are planning to get vaccinated. Your doctor will tell you if the vaccine you need is a live or attenuated vaccine or another type of vaccine. During treatment with Kesimpta, you should not receive live or attenuated vaccines, as they may cause an infection. Other types of vaccines may not work as well if received during treatment with Kesimpta.
Tell your doctor immediately if you experience any of the following symptoms during treatment with Kesimpta, as they may be signs of a serious disease:
- if you experience a rash, hives, difficulty breathing, swelling of the face, eyelids, lips, mouth, tongue, or throat, chest tightness, or feeling weak. These may be signs or symptoms of an allergic reaction.
- if you think your multiple sclerosis is getting worse (e.g., you experience weakness or changes in vision) or if you notice any new or unusual symptoms. These effects may be indicative of a rare brain disorder known as progressive multifocal leukoencephalopathy (PML), which is caused by a viral infection.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age, as Kesimpta has not been studied in this age group.
Other medicines and Kesimpta
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor or pharmacist:
- if you are taking, have recently taken, or might take medicines that affect the immune system. This is because the effects on the immune system may be added together.
- if you are planning to get vaccinated (see above "Warnings and Precautions").
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Pregnancy
You should avoid becoming pregnant while using Kesimpta and for 6 months after stopping treatment.
If you are a woman of childbearing potential, you should use an effective method of contraception during treatment and for 6 months after stopping treatment with Kesimpta. Talk to your doctor about the available options.
If you become pregnant or think you may be pregnant during treatment or within 6 months after the last dose, tell your doctor immediately. Your doctor will inform you of the potential risks of Kesimpta during pregnancy. This is because Kesimpta may reduce the number of immune cells (B lymphocytes) in both the mother and the fetus. Your doctor should report your pregnancy to Novartis. You can also report your pregnancy by contacting the local representative of Novartis (see section 6), in addition to contacting your doctor.
Breastfeeding
Kesimpta may pass into breast milk. Talk to your doctor about the benefits and risks before breastfeeding while using Kesimpta.
Vaccination of newborn babies
Ask your doctor or pharmacist before vaccinating your newborn baby if you used Kesimpta during pregnancy (see above "Warnings and Precautions").
Driving and using machines
Kesimpta is unlikely to affect your ability to drive or use machines.
Kesimpta contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
Kesimpta contains polysorbate 80
This medicine contains 0.08 mg of polysorbate 80 per dose. Polysorbates may cause allergic reactions. Tell your doctor if you have any known allergies.
3. How to use Kesimpta
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, ask your doctor or pharmacist again.
Kesimpta is given by subcutaneous injection (injection under the skin).
The first injection should be performed under the guidance of a healthcare professional.
The pre-filled pens of Kesimpta are for single use only.
You can find detailed instructions on how to inject Kesimpta in the section "Instructions for use of Kesimpta in Sensoready Pen" at the end of this leaflet.
'QR code to be included' + www.kesimpta.eu
You can use Kesimpta at any time of day (morning, afternoon, or evening).
How much Kesimpta and how often it is given
Do not exceed the dose that your doctor has prescribed.
- The initial dose is 20 mg of Kesimpta given on the first day of treatment (Week 0) and after 1 week and 2 weeks (Week 1 and Week 2). After these first 3 injections, no injection should be given the following week (Week 3).
- The recommended dose is 20 mg of Kesimpta once a month, starting from Week 4.
Time | Dose |
Week 0 (first day of treatment) | 20 mg |
Week 1 | 20 mg |
Week 2 | 20 mg |
Week 3 | No injection |
Week 4 | 20 mg |
Afterwards, every month | 20 mg |
How long to use Kesimpta
Continue using Kesimpta every month for as long as your doctor tells you.
Your doctor will regularly check the state of your disease to see if the treatment is having the desired effect.
If you have any doubts about how long you should use Kesimpta, ask your doctor, pharmacist, or nurse.
If you use more Kesimpta than you should
If too much Kesimpta has been injected, tell your doctor immediately.
If you forget to use Kesimpta
To get the full benefit of Kesimpta, it is important that you inject each dose when it is due.
If you have missed an injection of Kesimpta, you should inject it as soon as possible. Do not wait until the next scheduled dose. The timing of the next injections should then be calculated from the day you injected this dose and not based on the original calendar (see also the previous section "How much Kesimpta and how often it is given").
If you stop using Kesimpta
Do not stop using Kesimpta or change your dose without talking to your doctor first.
Some side effects may be related to low levels of B lymphocytes in the blood. After stopping treatment with Kesimpta, your B lymphocyte levels in the blood will gradually increase to normal levels. This may take several months, during which time you may still experience some of the side effects described in this leaflet.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may happen with Kesimpta. If any of these side effects get serious, talk to your doctor, pharmacist, or nurse.
Very common(may affect more than 1 in 10 people)
- upper respiratory tract infections, with symptoms such as sore throat and runny nose
- reactions related to the injection, such as fever, headache, muscle pain, chills, and fatigue - these usually appear within 24 hours after an injection of Kesimpta and mainly after the first injection
- urinary tract infections
- reactions at the injection site, such as redness, pain, itching, and swelling at the injection site
Common(may affect up to 1 in 10 people)
- decrease in the blood level of a protein known as immunoglobulin M, which helps protect against infections
- oral herpes
- nausea, vomiting (have been reported in association with injection-related reactions)
Frequency not known(cannot be estimated from the available data)
- allergic reactions, with symptoms such as rash, hives, difficulty breathing, swelling of the face, eyelids, lips, mouth, tongue, or throat, chest tightness, or feeling weak
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Kesimpta
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after CAD and on the label after EXP. The expiry date is the last day of the month shown.
Store the pre-filled pen(s) in the outer carton to protect from light. Store in a refrigerator (between 2°C and 8°C). Do not freeze.
If necessary, Kesimpta can be left out of the refrigerator for a single period of up to 7 days at room temperature (not above 30°C). If not used during this time, Kesimpta can be put back in the refrigerator for up to 7 days.
Do not use this medicine if you notice that the solution contains visible particles or is cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Kesimpta Composition
- The active ingredient is ofatumumab. Each pre-filled pen contains 20 mg of ofatumumab.
- The other components are L-arginine, sodium acetate trihydrate, sodium chloride, polysorbate 80 (E 433), disodium edetate dihydrate, hydrochloric acid (for pH adjustment), and water for injectable preparations.
Product Appearance and Container Contents
Kesimpta injectable solution is a solution that is between transparent and slightly opalescent, and between colorless and yellow-slightly brown.
Kesimpta is available in single-unit containers that contain 1 Sensoready pre-filled pen and in multiple-unit containers that consist of 3 boxes, with 1 Sensoready pre-filled pen in each.
Only some package sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλάδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κύπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: https://www.ema.europa.eu.




Instructions for Use of Kesimpta in Sensoready Pen
It is essential that you understand and follow these instructions for use before injecting Kesimpta. If you have any doubts, consult your doctor, pharmacist, or nurse before using Kesimpta for the first time.
Remember:
- Do not usethe pen if the box seal or the pen seal is broken. Keep the pen in the sealed box until you are ready to use it.
- Do not shakethe pen.
- If the pen is dropped, do not use itif it appears to be damaged or if the pen fell without the cap in place.
- Discard the used pen immediately after use. Do not reuse a pen. See the section "How should I dispose of the used Kesimpta Sensoready pen?" at the end of these Instructions for Use.
How should I store Kesimpta?
- Keep the pen box in the refrigerator between 2°C and 8°C.
- Keep the pen in its original packaging to protect it from light until you are ready to use it.
- Do not freezethe pen.
Keep Kesimpta out of sight and reach of children.
Parts of the Kesimpta Sensoready Pen (see Image A):
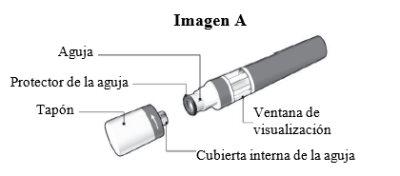
The Kesimpta Sensoready pen is shown with the cap removed. Do not remove the cap until you are ready for injection.
What you need for the injection:
Included in the box:
|
|
Not included in the box (see Image C):
|
|
See the section "How should I dispose of the used Kesimpta Sensoready pen?" at the end of these Instructions for Use.
Before the Injection:
Remove the pen from the refrigerator 15 to 30 minutes before the injectionto allow it to reach room temperature.
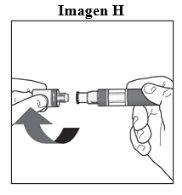
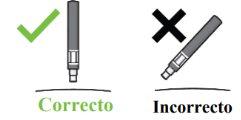
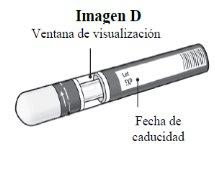
Step 1. Important Safety Checks Before Performing the Injection (see Image D):
Do not usethe pen if the liquid contains visible particles or is cloudy. You may see a small air bubble, which is normal.
Contact your pharmacist or healthcare professional if the pen does not meet any of these checks. |
|
|
|
Your Injection Step 4. Remove the Cap:
You may notice some medication dripping from the needle. This is normal. |
|
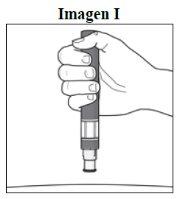
Step 5. Hold the Pen:
|
|
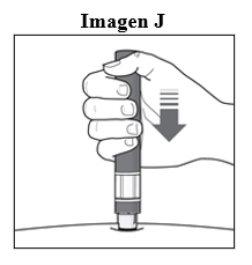
Step 6. Start the Injection:
|
|
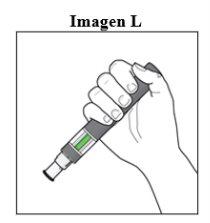
Step 7. Complete the Injection:
|
|
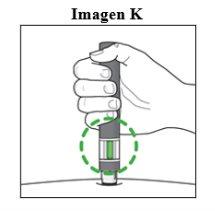
Important: During the Injectionyou will hear 2 loud clicks:
You must hold the pen firmly against the skin until the green indicatorfills the window and has stopped moving. |
After the Injection:
|
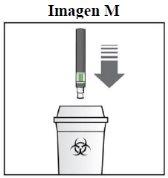
How should I dispose of the used Kesimpta Sensoready pen?
Step 8. Dispose of the Kesimpta Sensoready Pen:
Keep the sharps disposal container out of the reach of children. |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KESIMPTA 20 mg SOLUTION FOR INJECTION IN A PRE-FILLED PENDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE, 200 mgActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for KESIMPTA 20 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN
Discuss questions about KESIMPTA 20 mg SOLUTION FOR INJECTION IN A PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






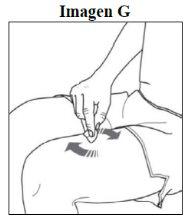
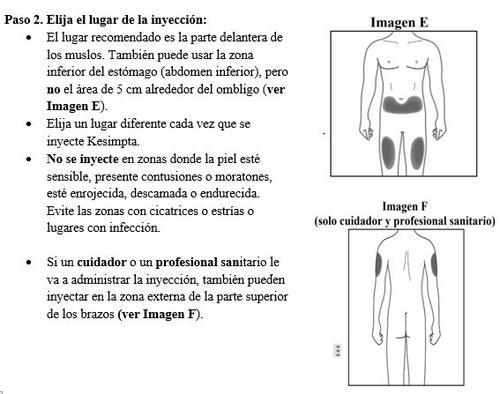
 Step 3. Clean the injection site:
Step 3. Clean the injection site: