
KALYDECO 75 mg granules in sachet

How to use KALYDECO 75 mg granules in sachet
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Kalydeco 13.4mg granules in sachet
Kalydeco 25 mg granules in sachet
Kalydeco 50 mg granules in sachet
Kalydeco 59.5mg granules in sachet
Kalydeco 75 mg granules in sachet
ivacaftor
Read this package leaflet carefully before your child starts taking this medicine, because it contains important information for your child.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your child's doctor or pharmacist.
- This medicine has been prescribed for your child only. Do not give it to others, even if they have the same symptoms as your child, as it may harm them.
- If your child experiences side effects, consult your child's doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Kalydeco and what is it used for
- What you need to know before your child takes Kalydeco
- How to take Kalydeco
- Possible side effects
- Storage of Kalydeco
- Contents of the pack and further information
1. What is Kalydeco and what is it used for
Kalydeco contains the active substance ivacaftor. Ivacaftor acts on the cystic fibrosis transmembrane conductance regulator (CFTR), a protein that forms a channel on the cell surface that allows particles such as chloride to enter and leave the cell. Due to mutations in the CFTRgene (see below), chloride movement is reduced in people with cystic fibrosis (CF). Ivacaftor helps certain abnormal CFTR proteins to open more frequently to improve chloride entry and exit from the cell.
Kalydeco granules are indicated:
- As monotherapy for the treatment of infants and children 1 month or older with a weight of 3 kg to less than 25 kg with cystic fibrosis (CF) and an R117Hmutation in the CFTRgene or one of the following gate opener mutations in the CFTRgene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549Nor S549R.
- In combination with ivacaftor/tezacaftor/elexacaftor granules in sachet for patients 2 to 6 years old who have CF, with at least one F508delmutation in the CFTRgene. If your child has been prescribed Kalydeco to take with ivacaftor/tezacaftor/elexacaftor, read the package leaflet of ivacaftor/tezacaftor/elexacaftor. It contains important information on how to take these two medicines.
2. What you need to know before your child takes Kalydeco
Do not give Kalydeco to your child
- if your child is allergic to ivacaftor or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your child's doctor before they start taking Kalydeco.
- Consult your child's doctor if your child has or has had liver problems in the past. It may be necessary for your child's doctor to adjust the dose for your child.
- An increase in liver enzymes in the blood has been observed in some people taking Kalydeco (alone or in combination with ivacaftor/tezacaftor/elexacaftor). Consult your child's doctor immediately if your child has any of the following symptoms, which may indicate liver problems:
- Pain or discomfort in the upper right side of the stomach (abdomen)
- Yellowing of the skin or whites of the eyes
- Loss of appetite
- Nausea or vomiting
- Dark urine
- Your child's doctor will perform blood tests to check how their liver is working before and during treatment, especially during the first year and especially if blood tests have indicated that they have had elevated liver enzymes in the past
- Depression (including suicidal thoughts and behaviors) has been reported in patients taking Kalydeco, mainly in combination with ivacaftor/tezacaftor/elexacaftor, which usually appears in the first three months of treatment. Consult your doctor immediately if you (or someone taking this medicine) experience any of the following symptoms: sad or changed mood, anxiety, feeling of emotional distress or self-harm or suicidal thoughts, which may be signs of depression.
- Consult your child's doctor if they have or have had kidney problems in the past.
- Kalydeco is not recommended for patients who have received an organ transplant.
- In some children and adolescents, an anomaly in the lens of the eye (cataract) has been observed without any effect on vision during treatment (alone or in combination with ivacaftor/tezacaftor/elexacaftor). Your child's doctor may perform some eye examinations before and during treatment.
- Kalydeco should only be used if your child has one of the mutations in the CFTRgene listed in section 1 (What is Kalydeco and what is it used for).
Children
This medicine should not be given to children under 1 month, as it is not known if ivacaftor is safe and effective in these children.
This medicine should not be given in combination with ivacaftor/tezacaftor/elexacaftor to children under 2 years, as it is not known if ivacaftor is safe and effective in them.
Other medicines and Kalydeco
Tell your child's doctor or pharmacist if your child is using, has recently used, or might use any other medicines. Some medicines may affect the way Kalydeco works or make it more likely to have side effects. In particular, tell your child's doctor if your child is taking any of the following medicines. Your child's doctor may decide to adjust the dose or that your child needs more check-ups.
- Antifungals(used to treat fungal infections). These include fluconazole, itraconazole, ketoconazole, posaconazole, and voriconazole.
- Antibiotics(used to treat bacterial infections). These include clarithromycin, erythromycin, rifabutin, rifampicin, and telithromycin.
- Medicines for epilepsy(used to treat seizures or epilepsy). These include carbamazepine, phenobarbital, and phenytoin.
- Herbal medicines. These include St. John's Wort (Hypericum perforatum).
- Immunosuppressants(used after organ transplantation). These include cyclosporin, everolimus, sirolimus, and tacrolimus.
- Cardiac glycosides(used to treat certain heart conditions). These include digoxin.
- Anticoagulants(used to prevent blood clots). These include warfarin.
- Medicines for diabetes. These include glimepiride and glipizide.
- Medicines to lower blood pressure. These include verapamil.
Taking Kalydeco with food and drinks
Avoid giving your child foods or drinks that contain grapefruit during treatment with Kalydeco, as they may increase the side effects of Kalydeco by increasing the amount of ivacaftor in your child's body.
Driving and using machines
Kalydeco may make your child dizzy. If your child feels dizzy, it is recommended that they do not ride a bike or perform any activity that requires their full attention.
Kalydeco contains lactose and sodium.
If your child's doctor has told your child that they have an intolerance to certain sugars, consult with them before your child takes this medicine.
Kalydeco contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to take Kalydeco
Follow the instructions for administration of this medicine exactly as prescribed by your child's doctor. If you are unsure, consult your child's doctor again.
Your child's doctor will determine the correct dose for your child. Your child should continue to use all their other medicines, unless their doctor tells them to stop taking any.
Table 1 provides the recommended dosing recommendations for Kalydeco.
Table1: Recommended dosing recommendations
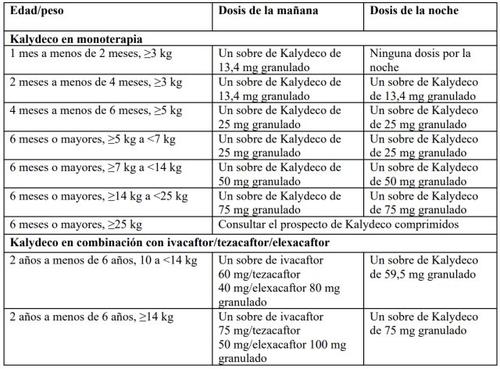
Administer the morning dose granules and the evening dose granules to your child 12 hours apart.
If your child has liver problems, it may be necessary for their doctor to reduce the dose of Kalydeco, as their liver may not eliminate the medicine from their body as quickly as in children with normal liver function.
- Moderate liver problems in children 6 months or older:the dose may be reduced to half of the recommended dose, i.e., one sachet once a day.
- Severe liver problems in children 6 months or older:it is not recommended, but your child's doctor will decide if it is suitable for your child to use this medicine, in which case the dose (as indicated in the table above) should be reduced to one sachet every two days.
- Liver problems in children between 1month and 6months of age:it is not recommended.
Kalydeco should be taken orally.
Each sachet is for single use only.
Administration of Kalydeco to your child:
- Hold the granule sachet with the cut line facing up.
- Gently shake the sachet to settle the contents.
- Open the sachet by tearing or cutting along the cut line.
- Mix the entire contents of one sachet with 5 ml of a soft food or liquid suitable for your child's age. The food or liquid should be at room temperature or below. Some examples of suitable soft foods or liquids for the age include fruit or vegetable puree, yogurt, apple sauce, water, milk, breast milk, formula milk, or juice.
- Once mixed, give the medicine to your child immediately. If this is not possible, give it within the next hour of mixing. Make sure your child takes the entire mixture immediately.
- Just before or just after administration, give your child a food or snack that contains fat (some examples are given below).
Fat-containing foods or snacks include those prepared with butter or oils or those that contain eggs. Other fat-containing foods are:
- Cheese, whole milk, whole milk dairy products, yogurt, breast milk, formula milk, chocolate
- Meat, oily fish
- Avocado, hummus (chickpea puree), soy products (tofu)
- Nuts, bars or nutritional drinks that contain fat
If your child takes more Kalydeco than they should
Your child may experience side effects, including those mentioned in section 4 below. If this happens, consult your child's doctor or pharmacist. If possible, show them your child's medicine and this package leaflet.
If you forget to give Kalydeco to your child
Give the missed dose if it has been less than 6 hours since the time your child was supposed to take the dose. Otherwise, wait until it is time for the next dose. Do not give a double dose to make up for missed doses.
If you interrupt treatment with Kalydeco for your child
Give Kalydeco to your child for as long as their doctor recommends. Do not stop treatment unless their doctor tells you to. If you have any further questions on the use of this medicine, ask your child's doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Stomach pain (abdominal) and increased liver enzymes in the blood.
Possible signs of liver problems
Increased liver enzymes in the blood are common in patients with CF and have also been reported in patients taking Kalydeco alone or in combination with ivacaftor/tezacaftor/elexacaftor.
In patients taking Kalydeco in combination with ivacaftor/tezacaftor/elexacaftor,liver damage and worsening of liver functionhave been reported in people with severe liver disease. Worsening of liver function can be severe and may require a transplant.
The following may be signs of liver problems:
- Pain or discomfort in the upper right side of the stomach (abdomen).
- Yellowing of the skin or whites of the eyes.
- Loss of appetite.
- Nausea or vomiting.
- Dark urine.
Depression
Signs of depression include a sad or changed mood, anxiety, or feeling of emotional distress.
Tell your child's doctor immediatelyif they experience any of these side effects.
Very common side effects(may affect more than 1 in 10 people)
- Upper respiratory tract infection (common cold), including sore throat and nasal congestion
- Headache
- Dizziness
- Diarrhea
- Stomach pain or abdominal pain
- Changes in the type of bacteria in the mucus
- Increased liver enzymes (signs of liver stress)
- Rash
Common side effects(may affect up to 1 in 10 people)
- Nasal congestion
- Ear pain, ear discomfort
- Ringing in the ears
- Redness in the ears
- Disorder of the inner ear (feeling of dizziness or spinning)
- Sinus problems (sinus congestion)
- Redness in the throat
- Breast lump
- Feeling sick (nausea)
- Flu
- Low blood sugar (hypoglycemia)
- Abnormal breathing (shortness of breath or difficulty breathing)
- Wind (flatulence)
- Spots (acne)
- Itching of the skin
- Increased creatine phosphokinase (sign of muscle breakdown), observed in blood tests
Uncommon side effects(may affect up to 1 in 100 people)
- Ear blockage
- Breast inflammation
- Breast enlargement in males
- Changes or pain in the nipples
- Wheezing
- Increased blood pressure
Frequency not known(cannot be estimated from the available data)
- Liver damage (liver injury)
- Increased bilirubin (liver blood test)
Additional side effects in children and adolescents
The side effects observed in children and adolescents are similar to those observed in adults. However, increased liver enzymes in the blood are more common in young children.
Reporting of side effects
If your child experiences any side effects, consult your child's doctor or pharmacist, even if they are not listed in this package leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Kalydeco
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, blister and sachet after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
Once mixed, the mixture has been shown to be stable for one hour.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Kalydeco Composition
Kalydeco 13.4 mg granules in sachet:
Each sachet contains 13.4 mg of ivacaftor.
Kalydeco 25 mg granules in sachet:
Each sachet contains 25 mg of ivacaftor.
Kalydeco 50 mg granules in sachet:
Each sachet contains 50 mg of ivacaftor.
Kalydeco 59.5 mg granules in sachet:
Each sachet contains 59.5 mg of ivacaftor.
Kalydeco 75 mg granules in sachet:
Each sachet contains 75 mg of ivacaftor.
The other components are: anhydrous colloidal silica, sodium croscarmellose, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, mannitol, sucralose, and sodium lauryl sulfate (E487).
See the end of section 2: Kalydeco contains lactose and sodium.
Product Appearance and Package Contents
Kalydeco 13.4 mg granules in sachet is a white to off-white granule.
Kalydeco 25 mg granules in sachet is a white to off-white granule.
Kalydeco 50 mg granules in sachet is a white to off-white granule.
Kalydeco 59.5 mg granules in sachet is a white to off-white granule.
Kalydeco 75 mg granules in sachet is a white to off-white granule.
The granules are supplied in sachets.
Kalydeco 13.4 mg granules in sachet, Kalydeco 25 mg granules in sachets, Kalydeco 50 mg granules in sachets, and Kalydeco 75 mg granules in sachet:
Package size of 56 sachets (contains 4 individual cartons with 14 sachets each).
Kalydeco 13.4 mg granules in sachet, Kalydeco 59.5 mg granules in sachet, and Kalydeco 75 mg granules in sachet:
Package size of 28 sachets (contains 4 individual cartons with 7 sachets each).
Marketing Authorization Holder
Vertex Pharmaceuticals (Ireland) Limited
Unit 49, Block 5, Northwood Court, Northwood Crescent
Dublin 9, D09 T665,
Ireland
Tel.: +353 (0)1 761 7299
Manufacturer
Almac Pharma Services (Ireland) Limited
Finnabair Industrial Estate
Dundalk
Co. Louth
A91 P9KD
Ireland
Almac Pharma Services Limited
Seagoe Industrial Estate
Craigavon
County Armagh
BT63 5UA
United Kingdom
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
|
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KALYDECO 75 mg granules in sachetDosage form: TABLET, 150 mgActive substance: ivacaftorManufacturer: Vertex Pharmaceuticals (Ireland) LimitedPrescription requiredDosage form: TABLET, 150 mgActive substance: ivacaftorManufacturer: Vertex Pharmaceuticals (Ireland) LimitedPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 25 mgActive substance: ivacaftorManufacturer: Vertex Pharmaceuticals (Ireland) LimitedPrescription required
Online doctors for KALYDECO 75 mg granules in sachet
Discuss questions about KALYDECO 75 mg granules in sachet, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions

















